 Found 60 hits of Enzyme Inhibition Constant Data
Found 60 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50138157

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-ch...)Show SMILES NCc1ccc(Cl)c(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H29ClN4O3/c29-23-14-9-19(17-30)16-25(23)31-26(34)18-33-15-5-4-8-24(28(33)36)32-27(35)22-12-10-21(11-13-22)20-6-2-1-3-7-20/h1-3,6-7,9-14,16,24H,4-5,8,15,17-18,30H2,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50138154

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-me...)Show SMILES Cc1ccc(CN)cc1NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C29H32N4O3/c1-20-10-11-21(18-30)17-26(20)31-27(34)19-33-16-6-5-9-25(29(33)36)32-28(35)24-14-12-23(13-15-24)22-7-3-2-4-8-22/h2-4,7-8,10-15,17,25H,5-6,9,16,18-19,30H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138156

(Biphenyl-4-carboxylic acid {1-[(3-aminomethyl-phen...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H30N4O3/c29-18-20-7-6-10-24(17-20)30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h1-3,6-10,12-15,17,25H,4-5,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human tissue type plasminogen activator |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50138156

(Biphenyl-4-carboxylic acid {1-[(3-aminomethyl-phen...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H30N4O3/c29-18-20-7-6-10-24(17-20)30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h1-3,6-10,12-15,17,25H,4-5,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human activated protein C (APC) |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50138156

(Biphenyl-4-carboxylic acid {1-[(3-aminomethyl-phen...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H30N4O3/c29-18-20-7-6-10-24(17-20)30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h1-3,6-10,12-15,17,25H,4-5,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human plasmin |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50138156

(Biphenyl-4-carboxylic acid {1-[(3-aminomethyl-phen...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H30N4O3/c29-18-20-7-6-10-24(17-20)30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h1-3,6-10,12-15,17,25H,4-5,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human Coagulation factor X |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138156

(Biphenyl-4-carboxylic acid {1-[(3-aminomethyl-phen...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H30N4O3/c29-18-20-7-6-10-24(17-20)30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h1-3,6-10,12-15,17,25H,4-5,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human urokinase-type plasminogen activator |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50138156

(Biphenyl-4-carboxylic acid {1-[(3-aminomethyl-phen...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H30N4O3/c29-18-20-7-6-10-24(17-20)30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h1-3,6-10,12-15,17,25H,4-5,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human trypsin |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50138156

(Biphenyl-4-carboxylic acid {1-[(3-aminomethyl-phen...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H30N4O3/c29-18-20-7-6-10-24(17-20)30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h1-3,6-10,12-15,17,25H,4-5,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human alpha-thrombin (FIIa) |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50138161
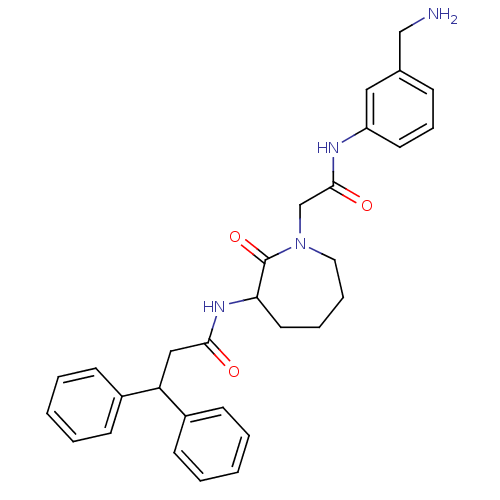
(CHEMBL321466 | N-{1-[(3-Aminomethyl-phenylcarbamoy...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)CC(c3ccccc3)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C30H34N4O3/c31-20-22-10-9-15-25(18-22)32-29(36)21-34-17-8-7-16-27(30(34)37)33-28(35)19-26(23-11-3-1-4-12-23)24-13-5-2-6-14-24/h1-6,9-15,18,26-27H,7-8,16-17,19-21,31H2,(H,32,36)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human trypsin |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50138161

(CHEMBL321466 | N-{1-[(3-Aminomethyl-phenylcarbamoy...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)CC(c3ccccc3)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C30H34N4O3/c31-20-22-10-9-15-25(18-22)32-29(36)21-34-17-8-7-16-27(30(34)37)33-28(35)19-26(23-11-3-1-4-12-23)24-13-5-2-6-14-24/h1-6,9-15,18,26-27H,7-8,16-17,19-21,31H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human plasmin |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50138161

(CHEMBL321466 | N-{1-[(3-Aminomethyl-phenylcarbamoy...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)CC(c3ccccc3)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C30H34N4O3/c31-20-22-10-9-15-25(18-22)32-29(36)21-34-17-8-7-16-27(30(34)37)33-28(35)19-26(23-11-3-1-4-12-23)24-13-5-2-6-14-24/h1-6,9-15,18,26-27H,7-8,16-17,19-21,31H2,(H,32,36)(H,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human alpha-thrombin (FIIa) |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50138161

(CHEMBL321466 | N-{1-[(3-Aminomethyl-phenylcarbamoy...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)CC(c3ccccc3)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C30H34N4O3/c31-20-22-10-9-15-25(18-22)32-29(36)21-34-17-8-7-16-27(30(34)37)33-28(35)19-26(23-11-3-1-4-12-23)24-13-5-2-6-14-24/h1-6,9-15,18,26-27H,7-8,16-17,19-21,31H2,(H,32,36)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human Coagulation factor X |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138161

(CHEMBL321466 | N-{1-[(3-Aminomethyl-phenylcarbamoy...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)CC(c3ccccc3)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C30H34N4O3/c31-20-22-10-9-15-25(18-22)32-29(36)21-34-17-8-7-16-27(30(34)37)33-28(35)19-26(23-11-3-1-4-12-23)24-13-5-2-6-14-24/h1-6,9-15,18,26-27H,7-8,16-17,19-21,31H2,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human tissue type plasminogen activator |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50138161

(CHEMBL321466 | N-{1-[(3-Aminomethyl-phenylcarbamoy...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)CC(c3ccccc3)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C30H34N4O3/c31-20-22-10-9-15-25(18-22)32-29(36)21-34-17-8-7-16-27(30(34)37)33-28(35)19-26(23-11-3-1-4-12-23)24-13-5-2-6-14-24/h1-6,9-15,18,26-27H,7-8,16-17,19-21,31H2,(H,32,36)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human activated protein C (APC) |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138161

(CHEMBL321466 | N-{1-[(3-Aminomethyl-phenylcarbamoy...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)CC(c3ccccc3)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C30H34N4O3/c31-20-22-10-9-15-25(18-22)32-29(36)21-34-17-8-7-16-27(30(34)37)33-28(35)19-26(23-11-3-1-4-12-23)24-13-5-2-6-14-24/h1-6,9-15,18,26-27H,7-8,16-17,19-21,31H2,(H,32,36)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human urokinase-type plasminogen activator |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50138161

(CHEMBL321466 | N-{1-[(3-Aminomethyl-phenylcarbamoy...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)CC(c3ccccc3)c3ccccc3)C2=O)c1 Show InChI InChI=1S/C30H34N4O3/c31-20-22-10-9-15-25(18-22)32-29(36)21-34-17-8-7-16-27(30(34)37)33-28(35)19-26(23-11-3-1-4-12-23)24-13-5-2-6-14-24/h1-6,9-15,18,26-27H,7-8,16-17,19-21,31H2,(H,32,36)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50138156

(Biphenyl-4-carboxylic acid {1-[(3-aminomethyl-phen...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H30N4O3/c29-18-20-7-6-10-24(17-20)30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h1-3,6-10,12-15,17,25H,4-5,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50138156

(Biphenyl-4-carboxylic acid {1-[(3-aminomethyl-phen...)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H30N4O3/c29-18-20-7-6-10-24(17-20)30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h1-3,6-10,12-15,17,25H,4-5,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >250 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50138157

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-ch...)Show SMILES NCc1ccc(Cl)c(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H29ClN4O3/c29-23-14-9-19(17-30)16-25(23)31-26(34)18-33-15-5-4-8-24(28(33)36)32-27(35)22-12-10-21(11-13-22)20-6-2-1-3-7-20/h1-3,6-7,9-14,16,24H,4-5,8,15,17-18,30H2,(H,31,34)(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human plasmin |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50138157

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-ch...)Show SMILES NCc1ccc(Cl)c(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H29ClN4O3/c29-23-14-9-19(17-30)16-25(23)31-26(34)18-33-15-5-4-8-24(28(33)36)32-27(35)22-12-10-21(11-13-22)20-6-2-1-3-7-20/h1-3,6-7,9-14,16,24H,4-5,8,15,17-18,30H2,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human trypsin |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50138154

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-me...)Show SMILES Cc1ccc(CN)cc1NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C29H32N4O3/c1-20-10-11-21(18-30)17-26(20)31-27(34)19-33-16-6-5-9-25(29(33)36)32-28(35)24-14-12-23(13-15-24)22-7-3-2-4-8-22/h2-4,7-8,10-15,17,25H,5-6,9,16,18-19,30H2,1H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human alpha-thrombin (FIIa) |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138154

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-me...)Show SMILES Cc1ccc(CN)cc1NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C29H32N4O3/c1-20-10-11-21(18-30)17-26(20)31-27(34)19-33-16-6-5-9-25(29(33)36)32-28(35)24-14-12-23(13-15-24)22-7-3-2-4-8-22/h2-4,7-8,10-15,17,25H,5-6,9,16,18-19,30H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human urokinase-type plasminogen activator |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50138154

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-me...)Show SMILES Cc1ccc(CN)cc1NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C29H32N4O3/c1-20-10-11-21(18-30)17-26(20)31-27(34)19-33-16-6-5-9-25(29(33)36)32-28(35)24-14-12-23(13-15-24)22-7-3-2-4-8-22/h2-4,7-8,10-15,17,25H,5-6,9,16,18-19,30H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human plasmin |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50138154

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-me...)Show SMILES Cc1ccc(CN)cc1NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C29H32N4O3/c1-20-10-11-21(18-30)17-26(20)31-27(34)19-33-16-6-5-9-25(29(33)36)32-28(35)24-14-12-23(13-15-24)22-7-3-2-4-8-22/h2-4,7-8,10-15,17,25H,5-6,9,16,18-19,30H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human Coagulation factor X |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50138154

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-me...)Show SMILES Cc1ccc(CN)cc1NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C29H32N4O3/c1-20-10-11-21(18-30)17-26(20)31-27(34)19-33-16-6-5-9-25(29(33)36)32-28(35)24-14-12-23(13-15-24)22-7-3-2-4-8-22/h2-4,7-8,10-15,17,25H,5-6,9,16,18-19,30H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human activated protein C (APC) |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138154

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-me...)Show SMILES Cc1ccc(CN)cc1NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C29H32N4O3/c1-20-10-11-21(18-30)17-26(20)31-27(34)19-33-16-6-5-9-25(29(33)36)32-28(35)24-14-12-23(13-15-24)22-7-3-2-4-8-22/h2-4,7-8,10-15,17,25H,5-6,9,16,18-19,30H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human tissue type plasminogen activator |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50138154

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-me...)Show SMILES Cc1ccc(CN)cc1NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C29H32N4O3/c1-20-10-11-21(18-30)17-26(20)31-27(34)19-33-16-6-5-9-25(29(33)36)32-28(35)24-14-12-23(13-15-24)22-7-3-2-4-8-22/h2-4,7-8,10-15,17,25H,5-6,9,16,18-19,30H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human trypsin |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138157

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-ch...)Show SMILES NCc1ccc(Cl)c(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H29ClN4O3/c29-23-14-9-19(17-30)16-25(23)31-26(34)18-33-15-5-4-8-24(28(33)36)32-27(35)22-12-10-21(11-13-22)20-6-2-1-3-7-20/h1-3,6-7,9-14,16,24H,4-5,8,15,17-18,30H2,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >870 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human urokinase-type plasminogen activator |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50138157

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-ch...)Show SMILES NCc1ccc(Cl)c(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H29ClN4O3/c29-23-14-9-19(17-30)16-25(23)31-26(34)18-33-15-5-4-8-24(28(33)36)32-27(35)22-12-10-21(11-13-22)20-6-2-1-3-7-20/h1-3,6-7,9-14,16,24H,4-5,8,15,17-18,30H2,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >870 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human alpha-thrombin (FIIa) |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50138157

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-ch...)Show SMILES NCc1ccc(Cl)c(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H29ClN4O3/c29-23-14-9-19(17-30)16-25(23)31-26(34)18-33-15-5-4-8-24(28(33)36)32-27(35)22-12-10-21(11-13-22)20-6-2-1-3-7-20/h1-3,6-7,9-14,16,24H,4-5,8,15,17-18,30H2,(H,31,34)(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >870 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human tissue type plasminogen activator |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50138157

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-ch...)Show SMILES NCc1ccc(Cl)c(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H29ClN4O3/c29-23-14-9-19(17-30)16-25(23)31-26(34)18-33-15-5-4-8-24(28(33)36)32-27(35)22-12-10-21(11-13-22)20-6-2-1-3-7-20/h1-3,6-7,9-14,16,24H,4-5,8,15,17-18,30H2,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >870 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human activated protein C (APC) |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50138157

(Biphenyl-4-carboxylic acid {1-[(5-aminomethyl-2-ch...)Show SMILES NCc1ccc(Cl)c(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H29ClN4O3/c29-23-14-9-19(17-30)16-25(23)31-26(34)18-33-15-5-4-8-24(28(33)36)32-27(35)22-12-10-21(11-13-22)20-6-2-1-3-7-20/h1-3,6-7,9-14,16,24H,4-5,8,15,17-18,30H2,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >870 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human Coagulation factor X |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204029
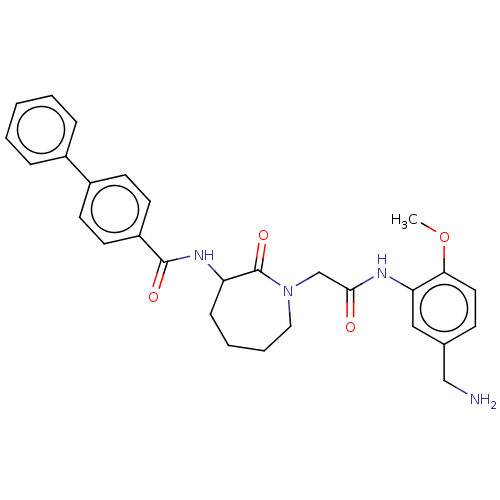
(CHEMBL419366)Show SMILES COc1ccc(CN)cc1NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C29H32N4O4/c1-37-26-15-10-20(18-30)17-25(26)31-27(34)19-33-16-6-5-9-24(29(33)36)32-28(35)23-13-11-22(12-14-23)21-7-3-2-4-8-21/h2-4,7-8,10-15,17,24H,5-6,9,16,18-19,30H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204638

(CHEMBL320473)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(Cc4ccccc4)cc3)C2=O)c1 Show InChI InChI=1S/C29H32N4O3/c30-19-23-9-6-10-25(18-23)31-27(34)20-33-16-5-4-11-26(29(33)36)32-28(35)24-14-12-22(13-15-24)17-21-7-2-1-3-8-21/h1-3,6-10,12-15,18,26H,4-5,11,16-17,19-20,30H2,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204631

(CHEMBL432789)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)C3CCCCC3)C2=O)c1 Show InChI InChI=1S/C28H36N4O3/c29-18-20-7-6-10-24(17-20)30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h6-7,10,12-15,17,21,25H,1-5,8-9,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204028

(CHEMBL104220)Show SMILES Cc1ccc(cc1)-c1ccc(cc1)C(=O)NC1CCCCN(CC(=O)Nc2cccc(CN)c2)C1=O Show InChI InChI=1S/C29H32N4O3/c1-20-8-10-22(11-9-20)23-12-14-24(15-13-23)28(35)32-26-7-2-3-16-33(29(26)36)19-27(34)31-25-6-4-5-21(17-25)18-30/h4-6,8-15,17,26H,2-3,7,16,18-19,30H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50206966
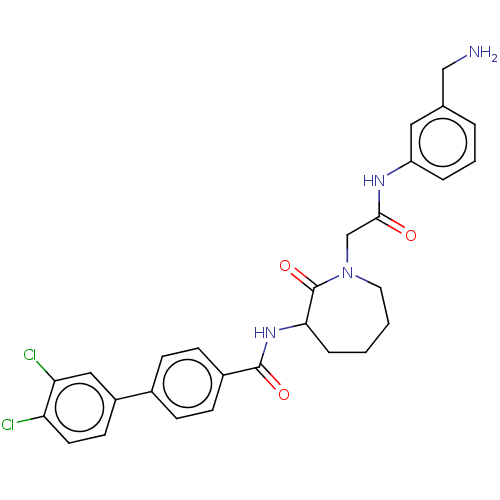
(CHEMBL322441)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccc(Cl)c(Cl)c3)C2=O)c1 Show InChI InChI=1S/C28H28Cl2N4O3/c29-23-12-11-21(15-24(23)30)19-7-9-20(10-8-19)27(36)33-25-6-1-2-13-34(28(25)37)17-26(35)32-22-5-3-4-18(14-22)16-31/h3-5,7-12,14-15,25H,1-2,6,13,16-17,31H2,(H,32,35)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204634
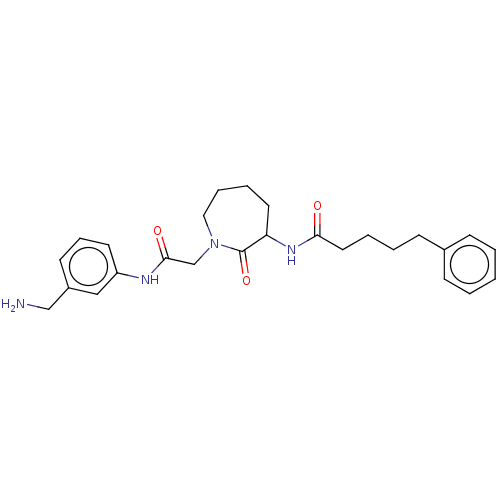
(CHEMBL320722)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)CCCCc3ccccc3)C2=O)c1 Show InChI InChI=1S/C26H34N4O3/c27-18-21-12-8-13-22(17-21)28-25(32)19-30-16-7-6-14-23(26(30)33)29-24(31)15-5-4-11-20-9-2-1-3-10-20/h1-3,8-10,12-13,17,23H,4-7,11,14-16,18-19,27H2,(H,28,32)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50207118

(CHEMBL320659)Show SMILES CCc1ccc(cc1)-c1ccc(cc1)C(=O)NC1CCCCN(CC(=O)Nc2cccc(CN)c2)C1=O Show InChI InChI=1S/C30H34N4O3/c1-2-21-9-11-23(12-10-21)24-13-15-25(16-14-24)29(36)33-27-8-3-4-17-34(30(27)37)20-28(35)32-26-7-5-6-22(18-26)19-31/h5-7,9-16,18,27H,2-4,8,17,19-20,31H2,1H3,(H,32,35)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204069
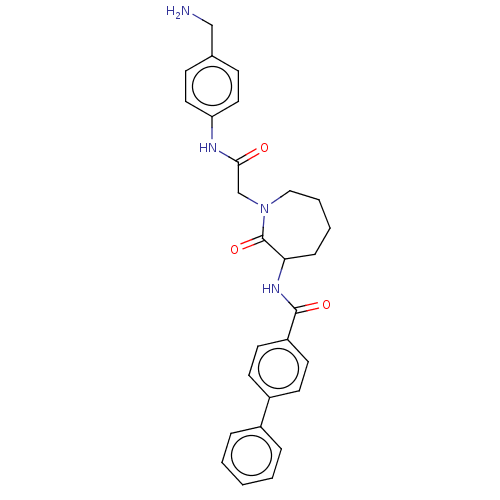
(CHEMBL107427)Show SMILES NCc1ccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)cc1 Show InChI InChI=1S/C28H30N4O3/c29-18-20-9-15-24(16-10-20)30-26(33)19-32-17-5-4-8-25(28(32)35)31-27(34)23-13-11-22(12-14-23)21-6-2-1-3-7-21/h1-3,6-7,9-16,25H,4-5,8,17-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50206960
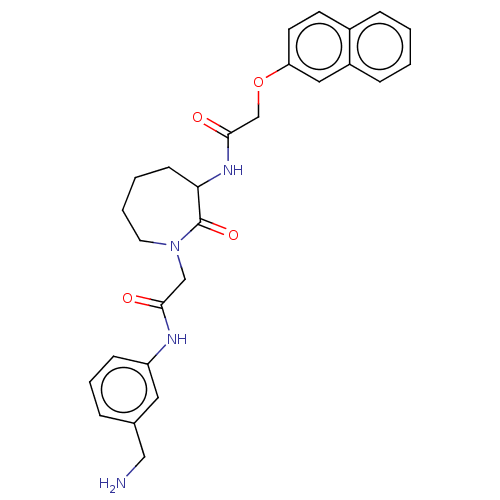
(CHEMBL106903)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)COc3ccc4ccccc4c3)C2=O)c1 Show InChI InChI=1S/C27H30N4O4/c28-16-19-6-5-9-22(14-19)29-25(32)17-31-13-4-3-10-24(27(31)34)30-26(33)18-35-23-12-11-20-7-1-2-8-21(20)15-23/h1-2,5-9,11-12,14-15,24H,3-4,10,13,16-18,28H2,(H,29,32)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204033

(CHEMBL317458)Show SMILES CCc1ccc(cc1)C(=O)NC1CCCCN(CC(=O)Nc2cccc(CN)c2)C1=O Show InChI InChI=1S/C24H30N4O3/c1-2-17-9-11-19(12-10-17)23(30)27-21-8-3-4-13-28(24(21)31)16-22(29)26-20-7-5-6-18(14-20)15-25/h5-7,9-12,14,21H,2-4,8,13,15-16,25H2,1H3,(H,26,29)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204044
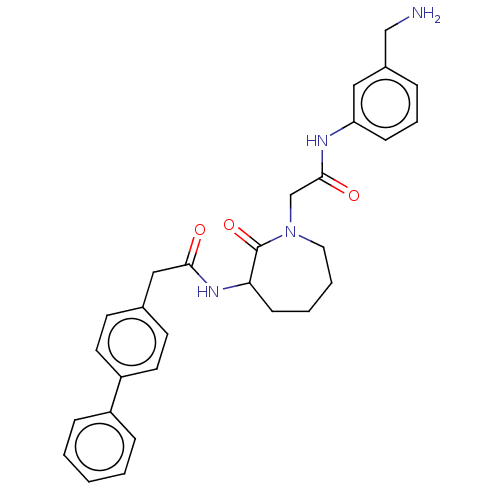
(CHEMBL104601)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)Cc3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C29H32N4O3/c30-19-22-7-6-10-25(17-22)31-28(35)20-33-16-5-4-11-26(29(33)36)32-27(34)18-21-12-14-24(15-13-21)23-8-2-1-3-9-23/h1-3,6-10,12-15,17,26H,4-5,11,16,18-20,30H2,(H,31,35)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204036

(CHEMBL326694)Show SMILES NCCc1ccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)cc1 Show InChI InChI=1S/C29H32N4O3/c30-18-17-21-9-15-25(16-10-21)31-27(34)20-33-19-5-4-8-26(29(33)36)32-28(35)24-13-11-23(12-14-24)22-6-2-1-3-7-22/h1-3,6-7,9-16,26H,4-5,8,17-20,30H2,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204943
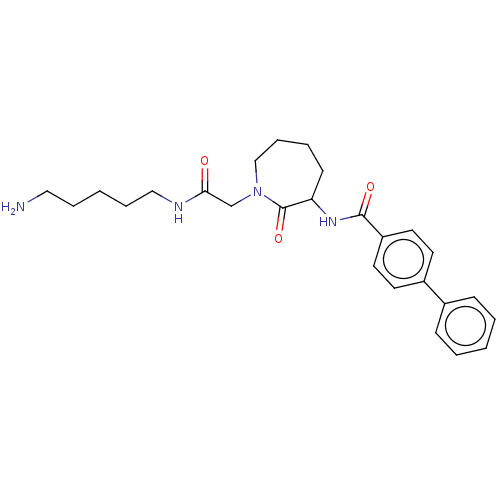
(CHEMBL104309)Show SMILES NCCCCCNC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C26H34N4O3/c27-16-6-2-7-17-28-24(31)19-30-18-8-5-11-23(26(30)33)29-25(32)22-14-12-21(13-15-22)20-9-3-1-4-10-20/h1,3-4,9-10,12-15,23H,2,5-8,11,16-19,27H2,(H,28,31)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204636

(CHEMBL106146)Show SMILES NCC1CCC(CC1)NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O |(20.56,-4.01,;19.52,-2.87,;18.02,-3.2,;16.74,-4.06,;15.48,-2.75,;14.24,-2.59,;15.52,-1.73,;16.7,-3.01,;12.75,-2.94,;11.7,-1.83,;12.14,-.35,;10.2,-2.17,;9.14,-1.06,;9.7,.39,;8.91,1.72,;7.39,1.93,;6.27,.88,;6.39,-.66,;5.06,-1.43,;3.74,-.66,;3.73,.88,;2.41,-1.44,;1.07,-.68,;-.26,-1.44,;-.26,-2.99,;1.07,-3.76,;2.41,-2.99,;-1.58,-3.76,;-2.93,-2.98,;-4.26,-3.75,;-4.26,-5.29,;-2.91,-6.06,;-1.58,-5.29,;7.67,-1.52,;7.46,-3.04,)| Show InChI InChI=1S/C28H36N4O3/c29-18-20-9-15-24(16-10-20)30-26(33)19-32-17-5-4-8-25(28(32)35)31-27(34)23-13-11-22(12-14-23)21-6-2-1-3-7-21/h1-3,6-7,11-14,20,24-25H,4-5,8-10,15-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204637
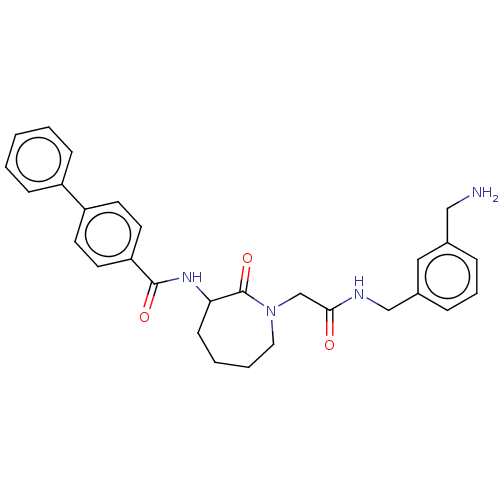
(CHEMBL319925)Show SMILES NCc1cccc(CNC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C29H32N4O3/c30-18-21-7-6-8-22(17-21)19-31-27(34)20-33-16-5-4-11-26(29(33)36)32-28(35)25-14-12-24(13-15-25)23-9-2-1-3-10-23/h1-3,6-10,12-15,17,26H,4-5,11,16,18-20,30H2,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204038
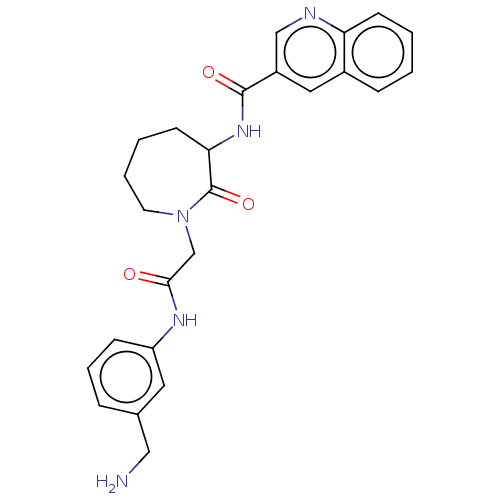
(CHEMBL107791)Show SMILES NCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3cnc4ccccc4c3)C2=O)c1 Show InChI InChI=1S/C25H27N5O3/c26-14-17-6-5-8-20(12-17)28-23(31)16-30-11-4-3-10-22(25(30)33)29-24(32)19-13-18-7-1-2-9-21(18)27-15-19/h1-2,5-9,12-13,15,22H,3-4,10-11,14,16,26H2,(H,28,31)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204641

(CHEMBL107095)Show SMILES O=C(CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O)Nc1ccc2CCNCc2c1 Show InChI InChI=1S/C30H32N4O3/c35-28(32-26-14-13-23-15-16-31-19-25(23)18-26)20-34-17-5-4-8-27(30(34)37)33-29(36)24-11-9-22(10-12-24)21-6-2-1-3-7-21/h1-3,6-7,9-14,18,27,31H,4-5,8,15-17,19-20H2,(H,32,35)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204633
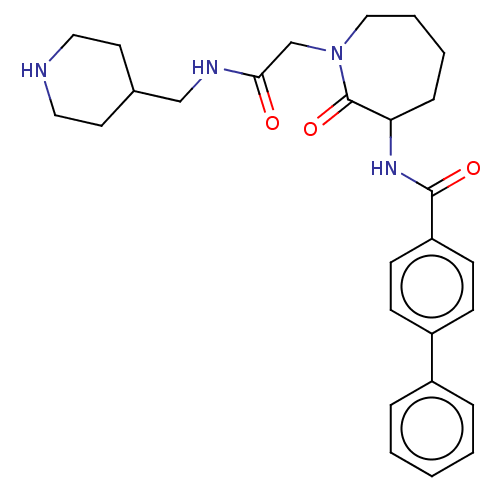
(CHEMBL107805)Show SMILES O=C(CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O)NCC1CCNCC1 Show InChI InChI=1S/C27H34N4O3/c32-25(29-18-20-13-15-28-16-14-20)19-31-17-5-4-8-24(27(31)34)30-26(33)23-11-9-22(10-12-23)21-6-2-1-3-7-21/h1-3,6-7,9-12,20,24,28H,4-5,8,13-19H2,(H,29,32)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204632

(CHEMBL321378)Show SMILES Nc1cccc(CNC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H30N4O3/c29-24-10-6-7-20(17-24)18-30-26(33)19-32-16-5-4-11-25(28(32)35)31-27(34)23-14-12-22(13-15-23)21-8-2-1-3-9-21/h1-3,6-10,12-15,17,25H,4-5,11,16,18-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204045

(CHEMBL106183)Show SMILES CCN(CC)Cc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C32H38N4O3/c1-3-35(4-2)22-24-11-10-14-28(21-24)33-30(37)23-36-20-9-8-15-29(32(36)39)34-31(38)27-18-16-26(17-19-27)25-12-6-5-7-13-25/h5-7,10-14,16-19,21,29H,3-4,8-9,15,20,22-23H2,1-2H3,(H,33,37)(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204635
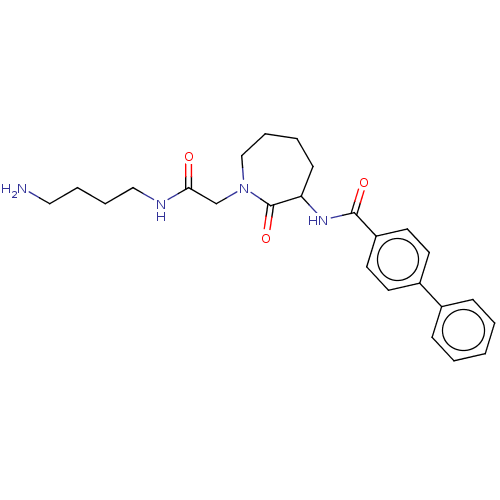
(CHEMBL107476)Show SMILES NCCCCNC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C25H32N4O3/c26-15-5-6-16-27-23(30)18-29-17-7-4-10-22(25(29)32)28-24(31)21-13-11-20(12-14-21)19-8-2-1-3-9-19/h1-3,8-9,11-14,22H,4-7,10,15-18,26H2,(H,27,30)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204027

(CHEMBL326448)Show SMILES NC(=O)c1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C28H28N4O4/c29-26(34)22-9-6-10-23(17-22)30-25(33)18-32-16-5-4-11-24(28(32)36)31-27(35)21-14-12-20(13-15-21)19-7-2-1-3-8-19/h1-3,6-10,12-15,17,24H,4-5,11,16,18H2,(H2,29,34)(H,30,33)(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204043
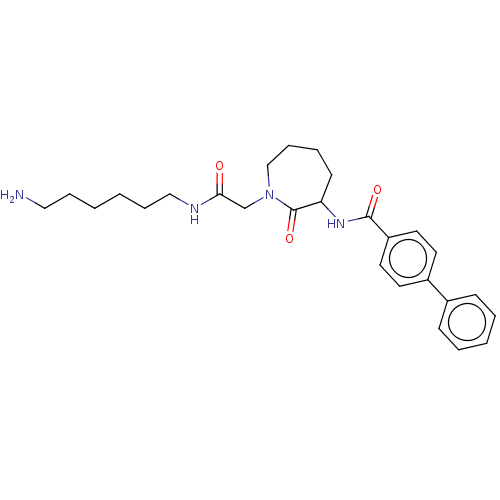
(CHEMBL107226)Show SMILES NCCCCCCNC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C27H36N4O3/c28-17-7-1-2-8-18-29-25(32)20-31-19-9-6-12-24(27(31)34)30-26(33)23-15-13-22(14-16-23)21-10-4-3-5-11-21/h3-5,10-11,13-16,24H,1-2,6-9,12,17-20,28H2,(H,29,32)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204640

(CHEMBL104354)Show SMILES NC1CCCc2ccc(NC(=O)CN3CCCCC(NC(=O)c4ccc(cc4)-c4ccccc4)C3=O)cc12 Show InChI InChI=1S/C31H34N4O3/c32-27-10-6-9-23-16-17-25(19-26(23)27)33-29(36)20-35-18-5-4-11-28(31(35)38)34-30(37)24-14-12-22(13-15-24)21-7-2-1-3-8-21/h1-3,7-8,12-17,19,27-28H,4-6,9-11,18,20,32H2,(H,33,36)(H,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204040

(CHEMBL107233)Show SMILES Nc1ccc(CNC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)cc1 Show InChI InChI=1S/C28H30N4O3/c29-24-15-9-20(10-16-24)18-30-26(33)19-32-17-5-4-8-25(28(32)35)31-27(34)23-13-11-22(12-14-23)21-6-2-1-3-7-21/h1-3,6-7,9-16,25H,4-5,8,17-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204621

(CHEMBL104434)Show SMILES NCc1ccccc1NC(=O)CN1CCCCC(NC(=O)c2ccc(cc2)-c2ccccc2)C1=O Show InChI InChI=1S/C28H30N4O3/c29-18-23-10-4-5-11-24(23)30-26(33)19-32-17-7-6-12-25(28(32)35)31-27(34)22-15-13-21(14-16-22)20-8-2-1-3-9-20/h1-5,8-11,13-16,25H,6-7,12,17-19,29H2,(H,30,33)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50204047

(CHEMBL107690)Show SMILES CNCc1cccc(NC(=O)CN2CCCCC(NC(=O)c3ccc(cc3)-c3ccccc3)C2=O)c1 Show InChI InChI=1S/C29H32N4O3/c1-30-19-21-8-7-11-25(18-21)31-27(34)20-33-17-6-5-12-26(29(33)36)32-28(35)24-15-13-23(14-16-24)22-9-3-2-4-10-22/h2-4,7-11,13-16,18,26,30H,5-6,12,17,19-20H2,1H3,(H,31,34)(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human tryptase. |
Bioorg Med Chem Lett 14: 309-12 (2003)
BindingDB Entry DOI: 10.7270/Q22F7MWG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data