 Found 55 hits of Enzyme Inhibition Constant Data
Found 55 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50136037
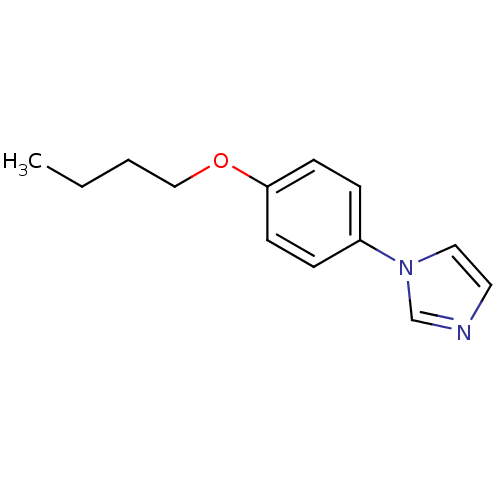
(1-(4-Butoxy-phenyl)-1H-imidazole | CHEMBL112532)Show InChI InChI=1S/C13H16N2O/c1-2-3-10-16-13-6-4-12(5-7-13)15-9-8-14-11-15/h4-9,11H,2-3,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50136037

(1-(4-Butoxy-phenyl)-1H-imidazole | CHEMBL112532)Show InChI InChI=1S/C13H16N2O/c1-2-3-10-16-13-6-4-12(5-7-13)15-9-8-14-11-15/h4-9,11H,2-3,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50138233

(2-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-2-10-19-15(4-1)5-3-13-21-17-8-6-16(7-9-17)20-12-11-18-14-20/h1-2,4,6-12,14H,3,5,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50138237

(3-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-3-15(13-18-9-1)4-2-12-21-17-7-5-16(6-8-17)20-11-10-19-14-20/h1,3,5-11,13-14H,2,4,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50138236
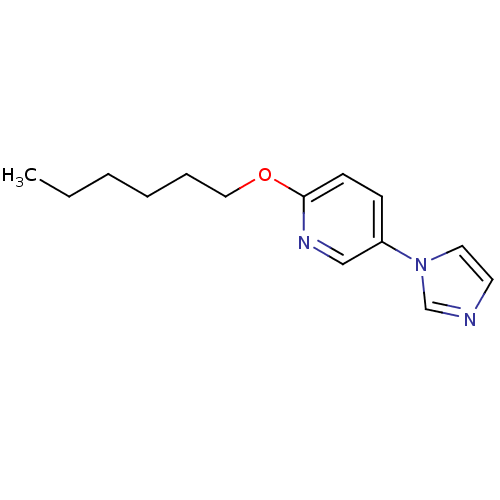
(2-Hexyloxy-5-imidazol-1-yl-pyridine | CHEMBL320105)Show InChI InChI=1S/C14H19N3O/c1-2-3-4-5-10-18-14-7-6-13(11-16-14)17-9-8-15-12-17/h6-9,11-12H,2-5,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138236

(2-Hexyloxy-5-imidazol-1-yl-pyridine | CHEMBL320105)Show InChI InChI=1S/C14H19N3O/c1-2-3-4-5-10-18-14-7-6-13(11-16-14)17-9-8-15-12-17/h6-9,11-12H,2-5,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138233

(2-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-2-10-19-15(4-1)5-3-13-21-17-8-6-16(7-9-17)20-12-11-18-14-20/h1-2,4,6-12,14H,3,5,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138232
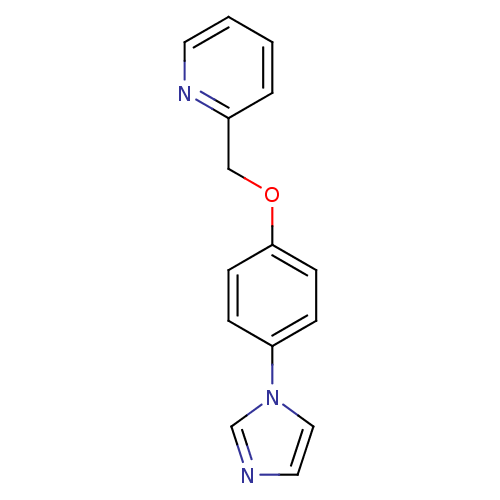
(2-(4-Imidazol-1-yl-phenoxymethyl)-pyridine | CHEMB...)Show InChI InChI=1S/C15H13N3O/c1-2-8-17-13(3-1)11-19-15-6-4-14(5-7-15)18-10-9-16-12-18/h1-10,12H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138237

(3-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-3-15(13-18-9-1)4-2-12-21-17-7-5-16(6-8-17)20-11-10-19-14-20/h1,3,5-11,13-14H,2,4,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138238

(4-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1(2-15-7-9-18-10-8-15)13-21-17-5-3-16(4-6-17)20-12-11-19-14-20/h3-12,14H,1-2,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50138238

(4-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1(2-15-7-9-18-10-8-15)13-21-17-5-3-16(4-6-17)20-12-11-19-14-20/h3-12,14H,1-2,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138231

(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-morpholine |...)Show InChI InChI=1S/C15H19N3O2/c1-3-15(4-2-14(1)18-6-5-16-13-18)20-12-9-17-7-10-19-11-8-17/h1-6,13H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50136037

(1-(4-Butoxy-phenyl)-1H-imidazole | CHEMBL112532)Show InChI InChI=1S/C13H16N2O/c1-2-3-10-16-13-6-4-12(5-7-13)15-9-8-14-11-15/h4-9,11H,2-3,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50138238

(4-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1(2-15-7-9-18-10-8-15)13-21-17-5-3-16(4-6-17)20-12-11-19-14-20/h3-12,14H,1-2,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50138232

(2-(4-Imidazol-1-yl-phenoxymethyl)-pyridine | CHEMB...)Show InChI InChI=1S/C15H13N3O/c1-2-8-17-13(3-1)11-19-15-6-4-14(5-7-15)18-10-9-16-12-18/h1-10,12H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50138233

(2-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-2-10-19-15(4-1)5-3-13-21-17-8-6-16(7-9-17)20-12-11-18-14-20/h1-2,4,6-12,14H,3,5,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50138232

(2-(4-Imidazol-1-yl-phenoxymethyl)-pyridine | CHEMB...)Show InChI InChI=1S/C15H13N3O/c1-2-8-17-13(3-1)11-19-15-6-4-14(5-7-15)18-10-9-16-12-18/h1-10,12H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50138237

(3-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-3-15(13-18-9-1)4-2-12-21-17-7-5-16(6-8-17)20-11-10-19-14-20/h1,3,5-11,13-14H,2,4,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138233

(2-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-2-10-19-15(4-1)5-3-13-21-17-8-6-16(7-9-17)20-12-11-18-14-20/h1-2,4,6-12,14H,3,5,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 252 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138238

(4-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1(2-15-7-9-18-10-8-15)13-21-17-5-3-16(4-6-17)20-12-11-19-14-20/h3-12,14H,1-2,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 344 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50136037

(1-(4-Butoxy-phenyl)-1H-imidazole | CHEMBL112532)Show InChI InChI=1S/C13H16N2O/c1-2-3-10-16-13-6-4-12(5-7-13)15-9-8-14-11-15/h4-9,11H,2-3,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 348 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138231

(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-morpholine |...)Show InChI InChI=1S/C15H19N3O2/c1-3-15(4-2-14(1)18-6-5-16-13-18)20-12-9-17-7-10-19-11-8-17/h1-6,13H,7-12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 399 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138234
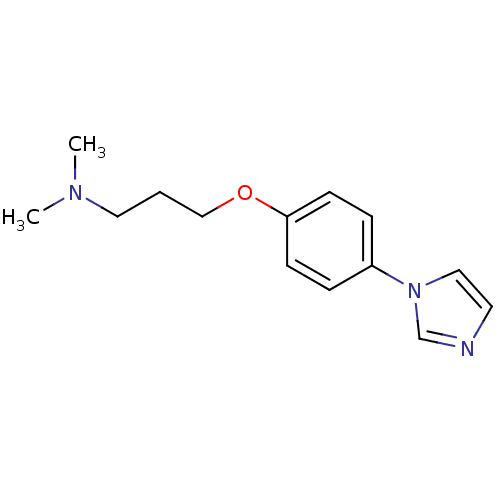
(CHEMBL114297 | [3-(4-Imidazol-1-yl-phenoxy)-propyl...)Show InChI InChI=1S/C14H19N3O/c1-16(2)9-3-11-18-14-6-4-13(5-7-14)17-10-8-15-12-17/h4-8,10,12H,3,9,11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138235

(CHEMBL113275 | [6-(4-Imidazol-1-yl-phenoxy)-hexyl]...)Show InChI InChI=1S/C17H25N3O/c1-19(2)12-5-3-4-6-14-21-17-9-7-16(8-10-17)20-13-11-18-15-20/h7-11,13,15H,3-6,12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138237

(3-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-3-15(13-18-9-1)4-2-12-21-17-7-5-16(6-8-17)20-11-10-19-14-20/h1,3,5-11,13-14H,2,4,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 417 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50136037

(1-(4-Butoxy-phenyl)-1H-imidazole | CHEMBL112532)Show InChI InChI=1S/C13H16N2O/c1-2-3-10-16-13-6-4-12(5-7-13)15-9-8-14-11-15/h4-9,11H,2-3,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138239
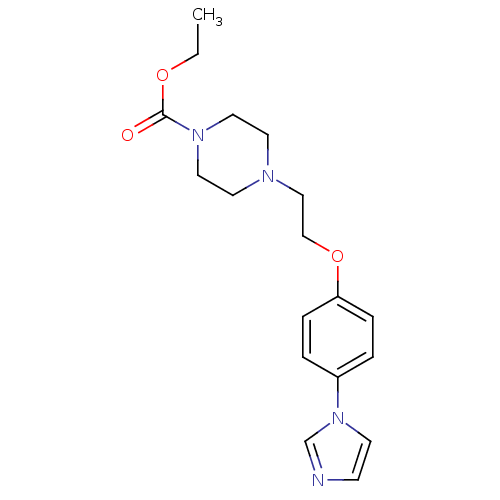
(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-piperazine-1...)Show InChI InChI=1S/C18H24N4O3/c1-2-24-18(23)21-11-9-20(10-12-21)13-14-25-17-5-3-16(4-6-17)22-8-7-19-15-22/h3-8,15H,2,9-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 469 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138233

(2-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-2-10-19-15(4-1)5-3-13-21-17-8-6-16(7-9-17)20-12-11-18-14-20/h1-2,4,6-12,14H,3,5,13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138232

(2-(4-Imidazol-1-yl-phenoxymethyl)-pyridine | CHEMB...)Show InChI InChI=1S/C15H13N3O/c1-2-8-17-13(3-1)11-19-15-6-4-14(5-7-15)18-10-9-16-12-18/h1-10,12H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50138235

(CHEMBL113275 | [6-(4-Imidazol-1-yl-phenoxy)-hexyl]...)Show InChI InChI=1S/C17H25N3O/c1-19(2)12-5-3-4-6-14-21-17-9-7-16(8-10-17)20-13-11-18-15-20/h7-11,13,15H,3-6,12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138236

(2-Hexyloxy-5-imidazol-1-yl-pyridine | CHEMBL320105)Show InChI InChI=1S/C14H19N3O/c1-2-3-4-5-10-18-14-7-6-13(11-16-14)17-9-8-15-12-17/h6-9,11-12H,2-5,10H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138232

(2-(4-Imidazol-1-yl-phenoxymethyl)-pyridine | CHEMB...)Show InChI InChI=1S/C15H13N3O/c1-2-8-17-13(3-1)11-19-15-6-4-14(5-7-15)18-10-9-16-12-18/h1-10,12H,11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138238

(4-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1(2-15-7-9-18-10-8-15)13-21-17-5-3-16(4-6-17)20-12-11-19-14-20/h3-12,14H,1-2,13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138240
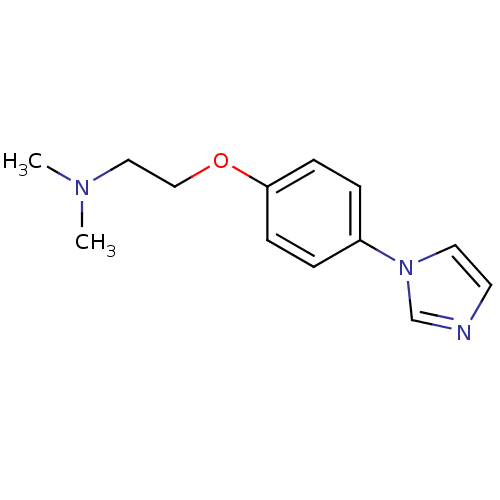
(CHEMBL325429 | [2-(4-Imidazol-1-yl-phenoxy)-ethyl]...)Show InChI InChI=1S/C13H17N3O/c1-15(2)9-10-17-13-5-3-12(4-6-13)16-8-7-14-11-16/h3-8,11H,9-10H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138237

(3-[3-(4-Imidazol-1-yl-phenoxy)-propyl]-pyridine | ...)Show InChI InChI=1S/C17H17N3O/c1-3-15(13-18-9-1)4-2-12-21-17-7-5-16(6-8-17)20-11-10-19-14-20/h1,3,5-11,13-14H,2,4,12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50138239

(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-piperazine-1...)Show InChI InChI=1S/C18H24N4O3/c1-2-24-18(23)21-11-9-20(10-12-21)13-14-25-17-5-3-16(4-6-17)22-8-7-19-15-22/h3-8,15H,2,9-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50138231

(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-morpholine |...)Show InChI InChI=1S/C15H19N3O2/c1-3-15(4-2-14(1)18-6-5-16-13-18)20-12-9-17-7-10-19-11-8-17/h1-6,13H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50138231

(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-morpholine |...)Show InChI InChI=1S/C15H19N3O2/c1-3-15(4-2-14(1)18-6-5-16-13-18)20-12-9-17-7-10-19-11-8-17/h1-6,13H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50138239

(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-piperazine-1...)Show InChI InChI=1S/C18H24N4O3/c1-2-24-18(23)21-11-9-20(10-12-21)13-14-25-17-5-3-16(4-6-17)22-8-7-19-15-22/h3-8,15H,2,9-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138240

(CHEMBL325429 | [2-(4-Imidazol-1-yl-phenoxy)-ethyl]...)Show InChI InChI=1S/C13H17N3O/c1-15(2)9-10-17-13-5-3-12(4-6-13)16-8-7-14-11-16/h3-8,11H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50138239

(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-piperazine-1...)Show InChI InChI=1S/C18H24N4O3/c1-2-24-18(23)21-11-9-20(10-12-21)13-14-25-17-5-3-16(4-6-17)22-8-7-19-15-22/h3-8,15H,2,9-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2D6 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50138234

(CHEMBL114297 | [3-(4-Imidazol-1-yl-phenoxy)-propyl...)Show InChI InChI=1S/C14H19N3O/c1-16(2)9-3-11-18-14-6-4-13(5-7-14)17-10-8-15-12-17/h4-8,10,12H,3,9,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138235

(CHEMBL113275 | [6-(4-Imidazol-1-yl-phenoxy)-hexyl]...)Show InChI InChI=1S/C17H25N3O/c1-19(2)12-5-3-4-6-14-21-17-9-7-16(8-10-17)20-13-11-18-15-20/h7-11,13,15H,3-6,12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138231

(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-morpholine |...)Show InChI InChI=1S/C15H19N3O2/c1-3-15(4-2-14(1)18-6-5-16-13-18)20-12-9-17-7-10-19-11-8-17/h1-6,13H,7-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138235

(CHEMBL113275 | [6-(4-Imidazol-1-yl-phenoxy)-hexyl]...)Show InChI InChI=1S/C17H25N3O/c1-19(2)12-5-3-4-6-14-21-17-9-7-16(8-10-17)20-13-11-18-15-20/h7-11,13,15H,3-6,12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50138234

(CHEMBL114297 | [3-(4-Imidazol-1-yl-phenoxy)-propyl...)Show InChI InChI=1S/C14H19N3O/c1-16(2)9-3-11-18-14-6-4-13(5-7-14)17-10-8-15-12-17/h4-8,10,12H,3,9,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C19 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50138240

(CHEMBL325429 | [2-(4-Imidazol-1-yl-phenoxy)-ethyl]...)Show InChI InChI=1S/C13H17N3O/c1-15(2)9-10-17-13-5-3-12(4-6-13)16-8-7-14-11-16/h3-8,11H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50138234

(CHEMBL114297 | [3-(4-Imidazol-1-yl-phenoxy)-propyl...)Show InChI InChI=1S/C14H19N3O/c1-16(2)9-3-11-18-14-6-4-13(5-7-14)17-10-8-15-12-17/h4-8,10,12H,3,9,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50138235

(CHEMBL113275 | [6-(4-Imidazol-1-yl-phenoxy)-hexyl]...)Show InChI InChI=1S/C17H25N3O/c1-19(2)12-5-3-4-6-14-21-17-9-7-16(8-10-17)20-13-11-18-15-20/h7-11,13,15H,3-6,12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138234

(CHEMBL114297 | [3-(4-Imidazol-1-yl-phenoxy)-propyl...)Show InChI InChI=1S/C14H19N3O/c1-16(2)9-3-11-18-14-6-4-13(5-7-14)17-10-8-15-12-17/h4-8,10,12H,3,9,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50138240

(CHEMBL325429 | [2-(4-Imidazol-1-yl-phenoxy)-ethyl]...)Show InChI InChI=1S/C13H17N3O/c1-15(2)9-10-17-13-5-3-12(4-6-13)16-8-7-14-11-16/h3-8,11H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138236

(2-Hexyloxy-5-imidazol-1-yl-pyridine | CHEMBL320105)Show InChI InChI=1S/C14H19N3O/c1-2-3-4-5-10-18-14-7-6-13(11-16-14)17-9-8-15-12-17/h6-9,11-12H,2-5,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 7.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138240

(CHEMBL325429 | [2-(4-Imidazol-1-yl-phenoxy)-ethyl]...)Show InChI InChI=1S/C13H17N3O/c1-15(2)9-10-17-13-5-3-12(4-6-13)16-8-7-14-11-16/h3-8,11H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50138236

(2-Hexyloxy-5-imidazol-1-yl-pyridine | CHEMBL320105)Show InChI InChI=1S/C14H19N3O/c1-2-3-4-5-10-18-14-7-6-13(11-16-14)17-9-8-15-12-17/h6-9,11-12H,2-5,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50138239

(4-[2-(4-Imidazol-1-yl-phenoxy)-ethyl]-piperazine-1...)Show InChI InChI=1S/C18H24N4O3/c1-2-24-18(23)21-11-9-20(10-12-21)13-14-25-17-5-3-16(4-6-17)22-8-7-19-15-22/h3-8,15H,2,9-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 3A4 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data