 Found 78 hits of Enzyme Inhibition Constant Data
Found 78 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140413
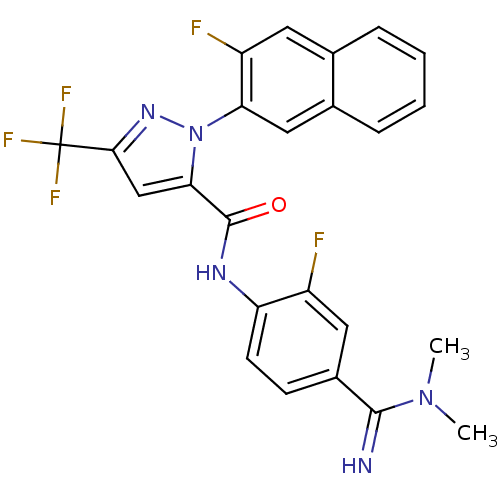
(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140424
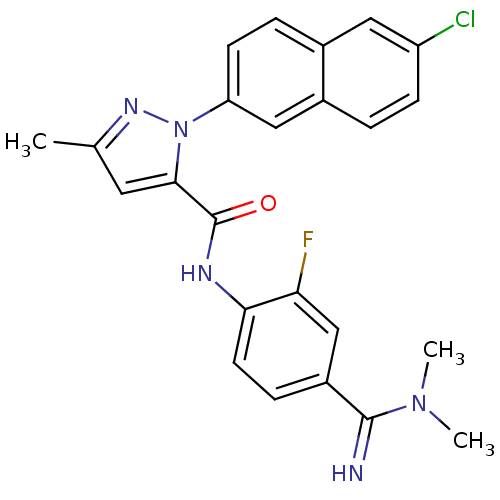
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140443
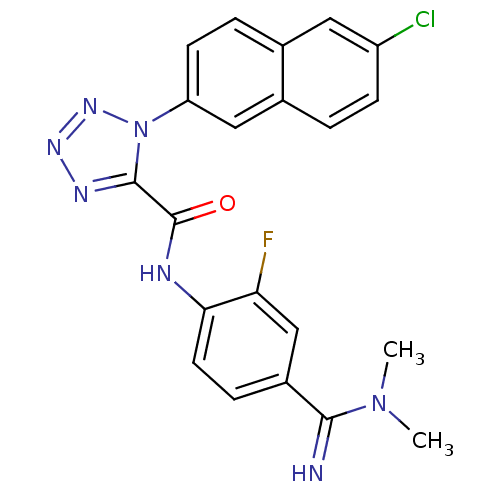
(1-(6-Chloro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2nnnn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C21H17ClFN7O/c1-29(2)19(24)14-5-8-18(17(23)11-14)25-21(31)20-26-27-28-30(20)16-7-4-12-9-15(22)6-3-13(12)10-16/h3-11,24H,1-2H3,(H,25,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140410
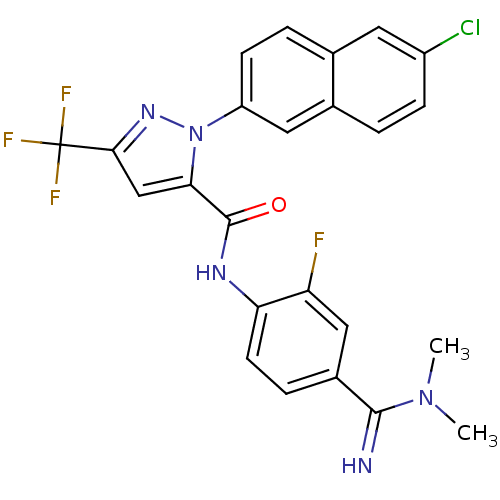
(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2ccc3cc(Cl)ccc3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18ClF4N5O/c1-33(2)22(30)15-5-8-19(18(26)11-15)31-23(35)20-12-21(24(27,28)29)32-34(20)17-7-4-13-9-16(25)6-3-14(13)10-17/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140422
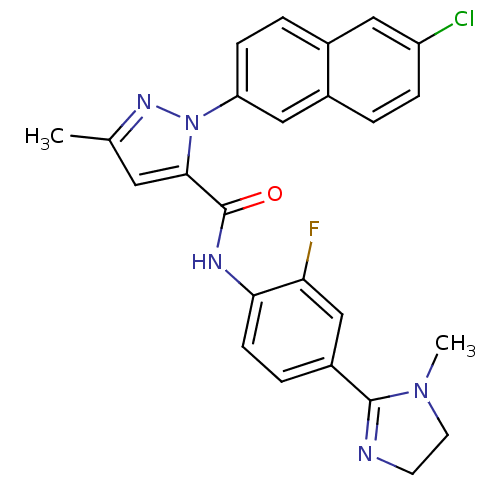
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 |c:4| Show InChI InChI=1S/C25H21ClFN5O/c1-15-11-23(32(30-15)20-7-4-16-12-19(26)6-3-17(16)13-20)25(33)29-22-8-5-18(14-21(22)27)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140415
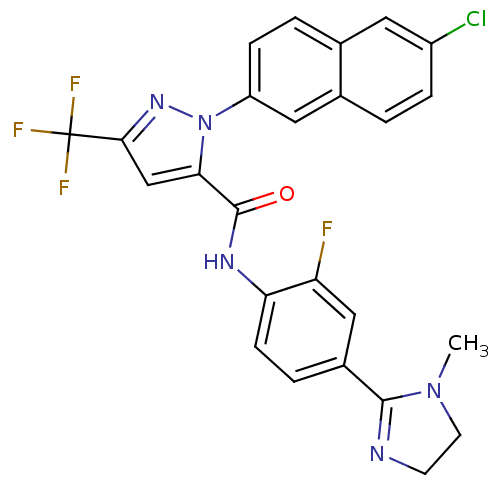
(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(nn2-c2ccc3cc(Cl)ccc3c2)C(F)(F)F)c(F)c1 |c:4| Show InChI InChI=1S/C25H18ClF4N5O/c1-34-9-8-31-23(34)16-4-7-20(19(27)12-16)32-24(36)21-13-22(25(28,29)30)33-35(21)18-6-3-14-10-17(26)5-2-15(14)11-18/h2-7,10-13H,8-9H2,1H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140418
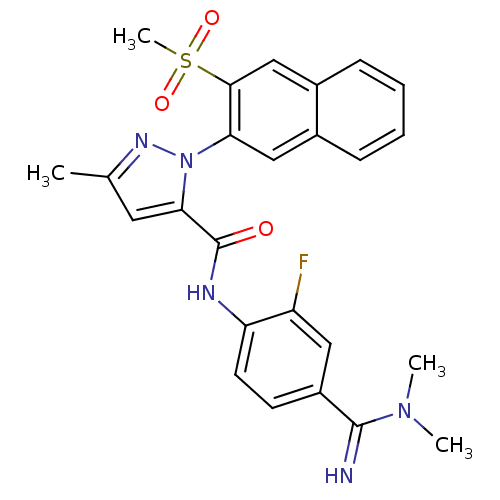
(2-(3-Methanesulfonyl-naphthalen-2-yl)-5-methyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2S(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C25H24FN5O3S/c1-15-11-22(25(32)28-20-10-9-18(12-19(20)26)24(27)30(2)3)31(29-15)21-13-16-7-5-6-8-17(16)14-23(21)35(4,33)34/h5-14,27H,1-4H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140409
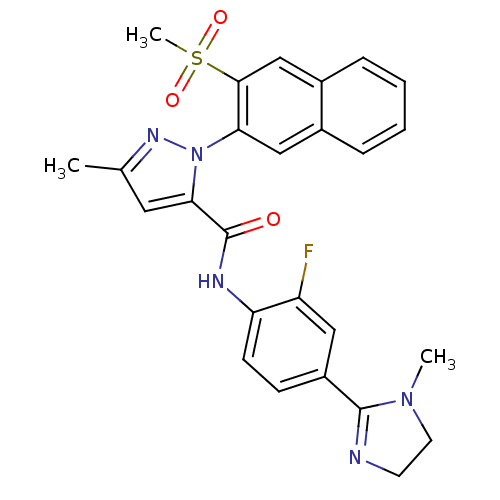
(2-(3-Methanesulfonyl-naphthalen-2-yl)-5-methyl-2H-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2S(C)(=O)=O)c(F)c1 |c:4| Show InChI InChI=1S/C26H24FN5O3S/c1-16-12-23(26(33)29-21-9-8-19(13-20(21)27)25-28-10-11-31(25)2)32(30-16)22-14-17-6-4-5-7-18(17)15-24(22)36(3,34)35/h4-9,12-15H,10-11H2,1-3H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140407
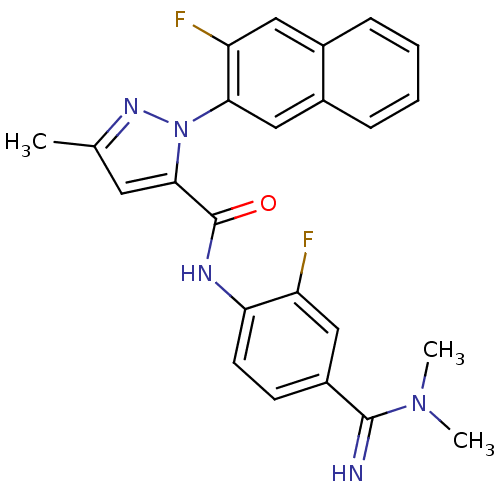
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C24H21F2N5O/c1-14-10-22(24(32)28-20-9-8-17(12-18(20)25)23(27)30(2)3)31(29-14)21-13-16-7-5-4-6-15(16)11-19(21)26/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140417
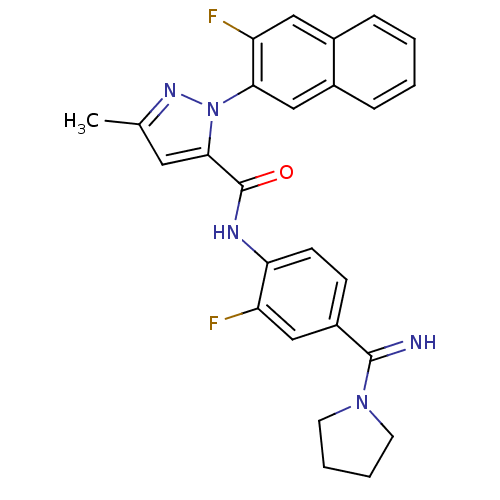
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCCC2)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C26H23F2N5O/c1-16-12-24(33(31-16)23-15-18-7-3-2-6-17(18)13-21(23)28)26(34)30-22-9-8-19(14-20(22)27)25(29)32-10-4-5-11-32/h2-3,6-9,12-15,29H,4-5,10-11H2,1H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140427
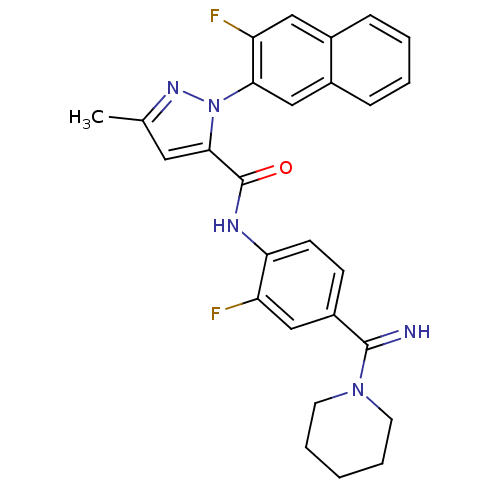
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCCCC2)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C27H25F2N5O/c1-17-13-25(34(32-17)24-16-19-8-4-3-7-18(19)14-22(24)29)27(35)31-23-10-9-20(15-21(23)28)26(30)33-11-5-2-6-12-33/h3-4,7-10,13-16,30H,2,5-6,11-12H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140416
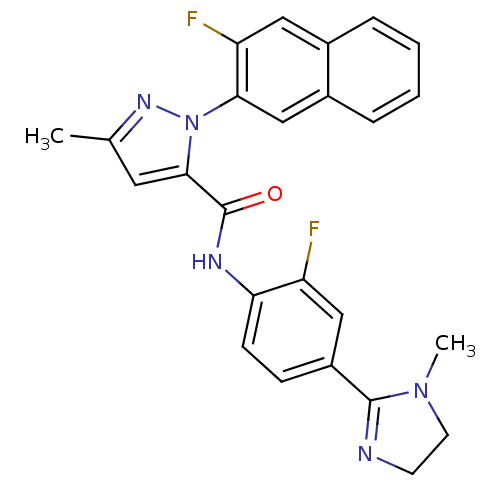
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 |c:4| Show InChI InChI=1S/C25H21F2N5O/c1-15-11-23(32(30-15)22-14-17-6-4-3-5-16(17)12-20(22)27)25(33)29-21-8-7-18(13-19(21)26)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140410

(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2ccc3cc(Cl)ccc3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18ClF4N5O/c1-33(2)22(30)15-5-8-19(18(26)11-15)31-23(35)20-12-21(24(27,28)29)32-34(20)17-7-4-13-9-16(25)6-3-14(13)10-17/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140434
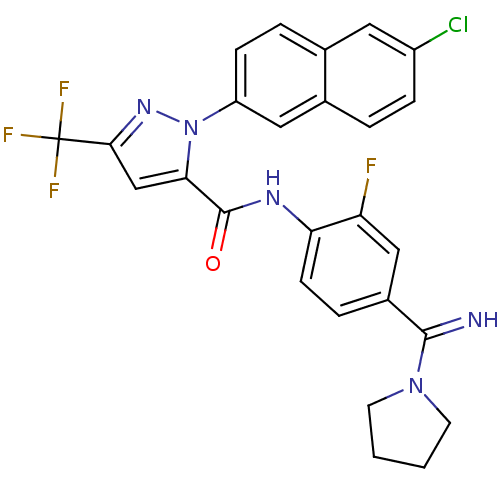
(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES Fc1cc(ccc1NC(=O)c1cc(nn1-c1ccc2cc(Cl)ccc2c1)C(F)(F)F)C(=N)N1CCCC1 Show InChI InChI=1S/C26H20ClF4N5O/c27-18-6-3-16-12-19(7-4-15(16)11-18)36-22(14-23(34-36)26(29,30)31)25(37)33-21-8-5-17(13-20(21)28)24(32)35-9-1-2-10-35/h3-8,11-14,32H,1-2,9-10H2,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140415

(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(nn2-c2ccc3cc(Cl)ccc3c2)C(F)(F)F)c(F)c1 |c:4| Show InChI InChI=1S/C25H18ClF4N5O/c1-34-9-8-31-23(34)16-4-7-20(19(27)12-16)32-24(36)21-13-22(25(28,29)30)33-35(21)18-6-3-14-10-17(26)5-2-15(14)11-18/h2-7,10-13H,8-9H2,1H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140443

(1-(6-Chloro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2nnnn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C21H17ClFN7O/c1-29(2)19(24)14-5-8-18(17(23)11-14)25-21(31)20-26-27-28-30(20)16-7-4-12-9-15(22)6-3-13(12)10-16/h3-11,24H,1-2H3,(H,25,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140413

(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140437
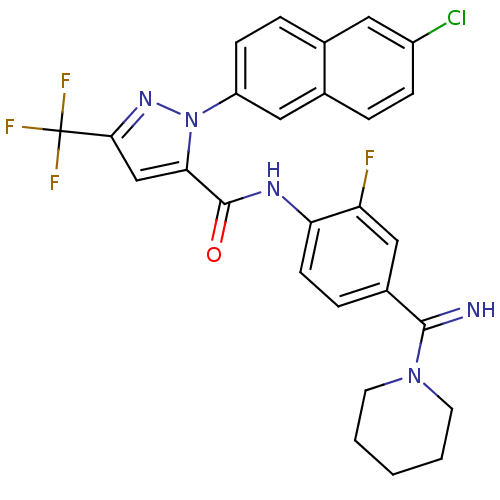
(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES Fc1cc(ccc1NC(=O)c1cc(nn1-c1ccc2cc(Cl)ccc2c1)C(F)(F)F)C(=N)N1CCCCC1 Show InChI InChI=1S/C27H22ClF4N5O/c28-19-7-4-17-13-20(8-5-16(17)12-19)37-23(15-24(35-37)27(30,31)32)26(38)34-22-9-6-18(14-21(22)29)25(33)36-10-2-1-3-11-36/h4-9,12-15,33H,1-3,10-11H2,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140424

(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140417

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCCC2)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C26H23F2N5O/c1-16-12-24(33(31-16)23-15-18-7-3-2-6-17(18)13-21(23)28)26(34)30-22-9-8-19(14-20(22)27)25(29)32-10-4-5-11-32/h2-3,6-9,12-15,29H,4-5,10-11H2,1H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140442
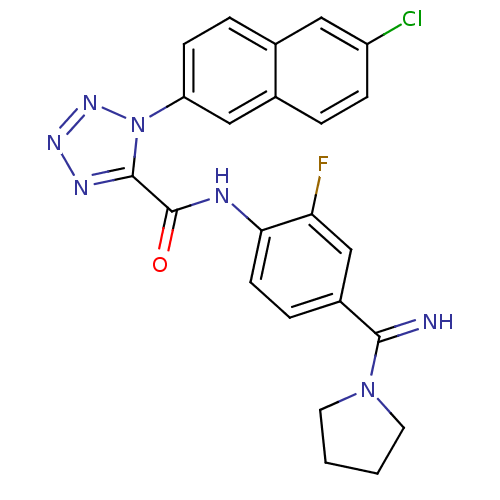
(1-(6-Chloro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES Fc1cc(ccc1NC(=O)c1nnnn1-c1ccc2cc(Cl)ccc2c1)C(=N)N1CCCC1 Show InChI InChI=1S/C23H19ClFN7O/c24-17-6-3-15-12-18(7-4-14(15)11-17)32-22(28-29-30-32)23(33)27-20-8-5-16(13-19(20)25)21(26)31-9-1-2-10-31/h3-8,11-13,26H,1-2,9-10H2,(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140446
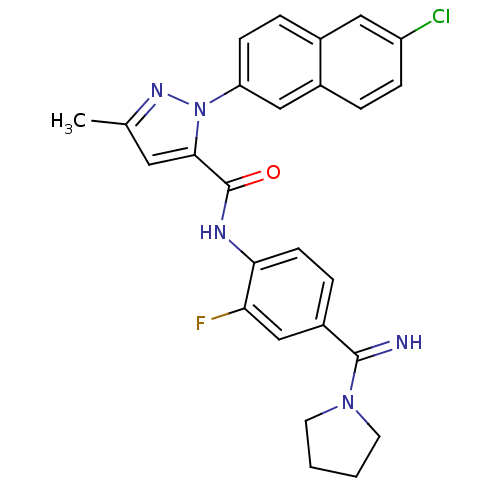
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCCC2)n(n1)-c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C26H23ClFN5O/c1-16-12-24(33(31-16)21-8-5-17-13-20(27)7-4-18(17)14-21)26(34)30-23-9-6-19(15-22(23)28)25(29)32-10-2-3-11-32/h4-9,12-15,29H,2-3,10-11H2,1H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140438
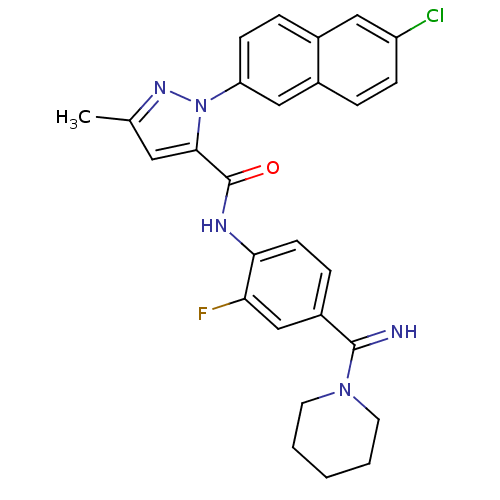
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCCCC2)n(n1)-c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C27H25ClFN5O/c1-17-13-25(34(32-17)22-9-6-18-14-21(28)8-5-19(18)15-22)27(35)31-24-10-7-20(16-23(24)29)26(30)33-11-3-2-4-12-33/h5-10,13-16,30H,2-4,11-12H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140427

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCCCC2)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C27H25F2N5O/c1-17-13-25(34(32-17)24-16-19-8-4-3-7-18(19)14-22(24)29)27(35)31-23-10-9-20(15-21(23)28)26(30)33-11-5-2-6-12-33/h3-4,7-10,13-16,30H,2,5-6,11-12H2,1H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140422

(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 |c:4| Show InChI InChI=1S/C25H21ClFN5O/c1-15-11-23(32(30-15)20-7-4-16-12-19(26)6-3-17(16)13-20)25(33)29-22-8-5-18(14-21(22)27)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140429
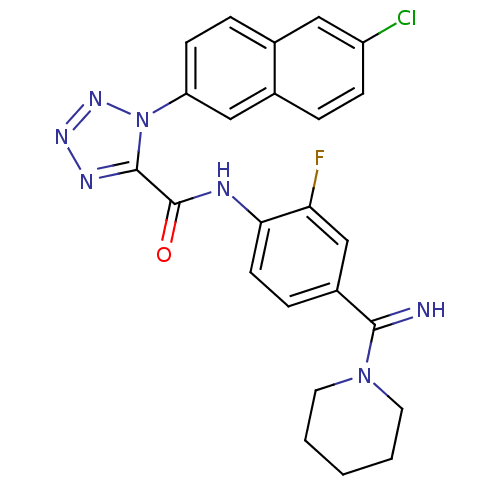
(1-(6-Chloro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES Fc1cc(ccc1NC(=O)c1nnnn1-c1ccc2cc(Cl)ccc2c1)C(=N)N1CCCCC1 Show InChI InChI=1S/C24H21ClFN7O/c25-18-7-4-16-13-19(8-5-15(16)12-18)33-23(29-30-31-33)24(34)28-21-9-6-17(14-20(21)26)22(27)32-10-2-1-3-11-32/h4-9,12-14,27H,1-3,10-11H2,(H,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50404324
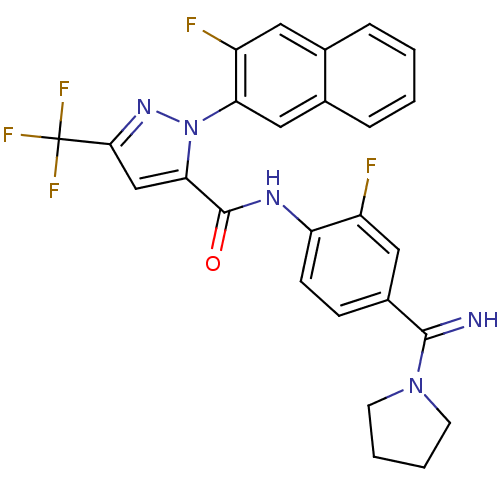
(CHEMBL2092985)Show SMILES Fc1cc(ccc1NC(=O)c1cc(nn1-c1cc2ccccc2cc1F)C(F)(F)F)C(=N)N1CCCC1 Show InChI InChI=1S/C26H20F5N5O/c27-18-12-17(24(32)35-9-3-4-10-35)7-8-20(18)33-25(37)22-14-23(26(29,30)31)34-36(22)21-13-16-6-2-1-5-15(16)11-19(21)28/h1-2,5-8,11-14,32H,3-4,9-10H2,(H,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140433
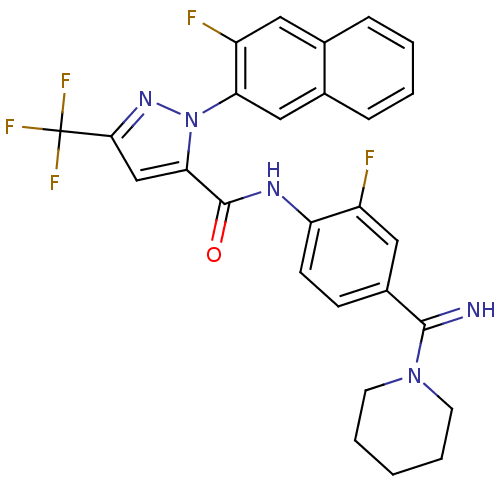
(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES Fc1cc(ccc1NC(=O)c1cc(nn1-c1cc2ccccc2cc1F)C(F)(F)F)C(=N)N1CCCCC1 Show InChI InChI=1S/C27H22F5N5O/c28-19-13-18(25(33)36-10-4-1-5-11-36)8-9-21(19)34-26(38)23-15-24(27(30,31)32)35-37(23)22-14-17-7-3-2-6-16(17)12-20(22)29/h2-3,6-9,12-15,33H,1,4-5,10-11H2,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140418

(2-(3-Methanesulfonyl-naphthalen-2-yl)-5-methyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2S(C)(=O)=O)c(F)c1 Show InChI InChI=1S/C25H24FN5O3S/c1-15-11-22(25(32)28-20-10-9-18(12-19(20)26)24(27)30(2)3)31(29-15)21-13-16-7-5-6-8-17(16)14-23(21)35(4,33)34/h5-14,27H,1-4H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140409

(2-(3-Methanesulfonyl-naphthalen-2-yl)-5-methyl-2H-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2S(C)(=O)=O)c(F)c1 |c:4| Show InChI InChI=1S/C26H24FN5O3S/c1-16-12-23(26(33)29-21-9-8-19(13-20(21)27)25-28-10-11-31(25)2)32(30-16)22-14-17-6-4-5-7-18(17)15-24(22)36(3,34)35/h4-9,12-15H,10-11H2,1-3H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140435
(1-(6-Chloro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2nnnn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 |c:4| Show InChI InChI=1S/C22H17ClFN7O/c1-30-9-8-25-20(30)15-4-7-19(18(24)12-15)26-22(32)21-27-28-29-31(21)17-6-3-13-10-16(23)5-2-14(13)11-17/h2-7,10-12H,8-9H2,1H3,(H,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140420
(2-(3-Methanesulfonyl-naphthalen-2-yl)-5-methyl-2H-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCCC2)n(n1)-c1cc2ccccc2cc1S(C)(=O)=O Show InChI InChI=1S/C27H26FN5O3S/c1-17-13-24(27(34)30-22-10-9-20(14-21(22)28)26(29)32-11-5-6-12-32)33(31-17)23-15-18-7-3-4-8-19(18)16-25(23)37(2,35)36/h3-4,7-10,13-16,29H,5-6,11-12H2,1-2H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140432
(1-(3-Fluoro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2nnnn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C21H17F2N7O/c1-29(2)19(24)14-7-8-17(15(22)10-14)25-21(31)20-26-27-28-30(20)18-11-13-6-4-3-5-12(13)9-16(18)23/h3-11,24H,1-2H3,(H,25,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140416

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 |c:4| Show InChI InChI=1S/C25H21F2N5O/c1-15-11-23(32(30-15)22-14-17-6-4-3-5-16(17)12-20(22)27)25(33)29-21-8-7-18(13-19(21)26)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140447
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)cc1 Show InChI InChI=1S/C24H22FN5O/c1-15-12-22(24(31)27-19-10-8-16(9-11-19)23(26)29(2)3)30(28-15)21-14-18-7-5-4-6-17(18)13-20(21)25/h4-14,26H,1-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140407

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C24H21F2N5O/c1-14-10-22(24(32)28-20-9-8-17(12-18(20)25)23(27)30(2)3)31(29-14)21-13-16-7-5-4-6-15(16)11-19(21)26/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140441
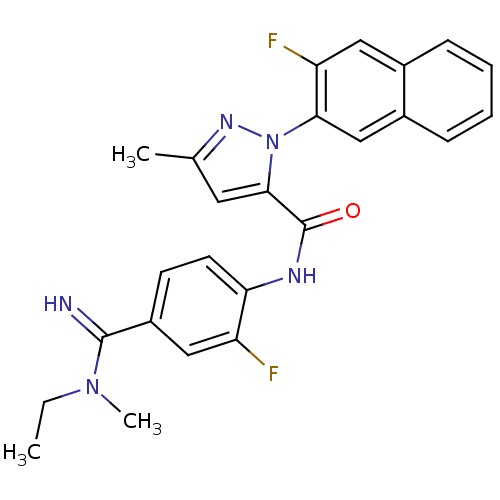
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CCN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C25H23F2N5O/c1-4-31(3)24(28)18-9-10-21(19(26)13-18)29-25(33)23-11-15(2)30-32(23)22-14-17-8-6-5-7-16(17)12-20(22)27/h5-14,28H,4H2,1-3H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140444
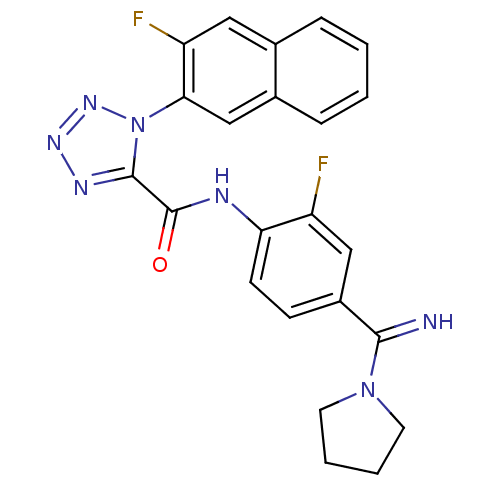
(1-(3-Fluoro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES Fc1cc(ccc1NC(=O)c1nnnn1-c1cc2ccccc2cc1F)C(=N)N1CCCC1 Show InChI InChI=1S/C23H19F2N7O/c24-17-12-16(21(26)31-9-3-4-10-31)7-8-19(17)27-23(33)22-28-29-30-32(22)20-13-15-6-2-1-5-14(15)11-18(20)25/h1-2,5-8,11-13,26H,3-4,9-10H2,(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
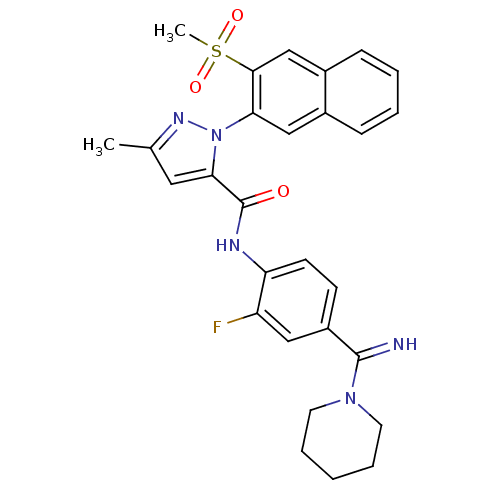
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140428
(2-(3-Methanesulfonyl-naphthalen-2-yl)-5-methyl-2H-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCCCC2)n(n1)-c1cc2ccccc2cc1S(C)(=O)=O Show InChI InChI=1S/C28H28FN5O3S/c1-18-14-25(34(32-18)24-16-19-8-4-5-9-20(19)17-26(24)38(2,36)37)28(35)31-23-11-10-21(15-22(23)29)27(30)33-12-6-3-7-13-33/h4-5,8-11,14-17,30H,3,6-7,12-13H2,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
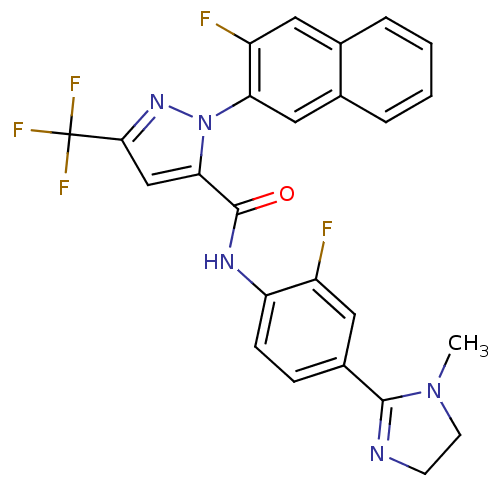
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140423
(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 |c:4| Show InChI InChI=1S/C25H18F5N5O/c1-34-9-8-31-23(34)16-6-7-19(17(26)11-16)32-24(36)21-13-22(25(28,29)30)33-35(21)20-12-15-5-3-2-4-14(15)10-18(20)27/h2-7,10-13H,8-9H2,1H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140411
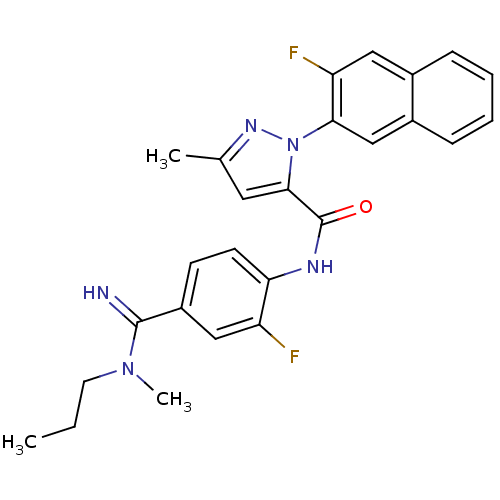
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CCCN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C26H25F2N5O/c1-4-11-32(3)25(29)19-9-10-22(20(27)14-19)30-26(34)24-12-16(2)31-33(24)23-15-18-8-6-5-7-17(18)13-21(23)28/h5-10,12-15,29H,4,11H2,1-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140430

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)CCN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C27H28F2N6O/c1-17-13-25(35(32-17)24-16-19-8-6-5-7-18(19)14-22(24)29)27(36)31-23-10-9-20(15-21(23)28)26(30)34(4)12-11-33(2)3/h5-10,13-16,30H,11-12H2,1-4H3,(H,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140439
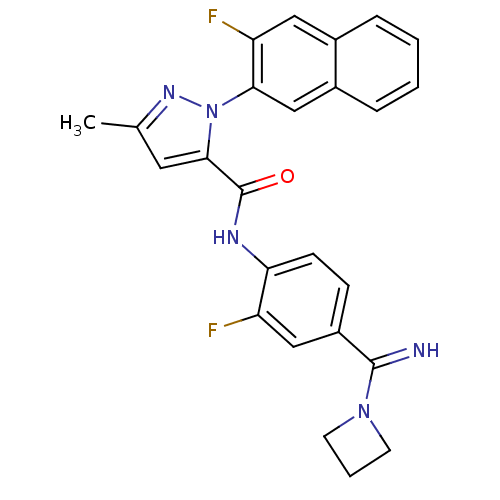
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCC2)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C25H21F2N5O/c1-15-11-23(32(30-15)22-14-17-6-3-2-5-16(17)12-20(22)27)25(33)29-21-8-7-18(13-19(21)26)24(28)31-9-4-10-31/h2-3,5-8,11-14,28H,4,9-10H2,1H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140421
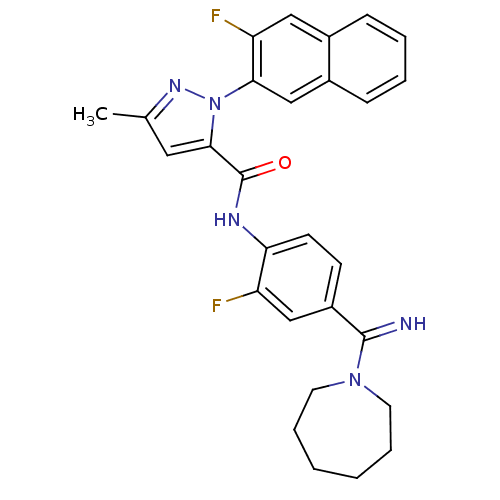
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCCCCC2)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C28H27F2N5O/c1-18-14-26(35(33-18)25-17-20-9-5-4-8-19(20)15-23(25)30)28(36)32-24-11-10-21(16-22(24)29)27(31)34-12-6-2-3-7-13-34/h4-5,8-11,14-17,31H,2-3,6-7,12-13H2,1H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140414
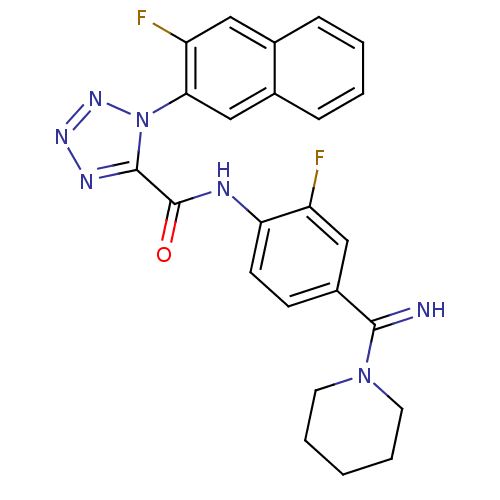
(1-(3-Fluoro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES Fc1cc(ccc1NC(=O)c1nnnn1-c1cc2ccccc2cc1F)C(=N)N1CCCCC1 Show InChI InChI=1S/C24H21F2N7O/c25-18-13-17(22(27)32-10-4-1-5-11-32)8-9-20(18)28-24(34)23-29-30-31-33(23)21-14-16-7-3-2-6-15(16)12-19(21)26/h2-3,6-9,12-14,27H,1,4-5,10-11H2,(H,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140425

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(Cc1ccccc1)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C30H25F2N5O/c1-19-14-28(37(35-19)27-17-22-11-7-6-10-21(22)15-25(27)32)30(38)34-26-13-12-23(16-24(26)31)29(33)36(2)18-20-8-4-3-5-9-20/h3-17,33H,18H2,1-2H3,(H,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140445
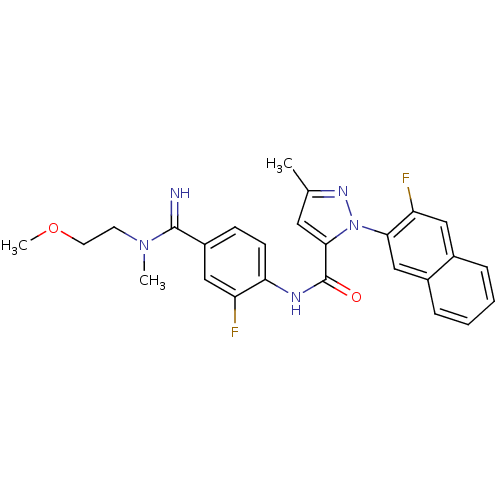
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES COCCN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C26H25F2N5O2/c1-16-12-24(33(31-16)23-15-18-7-5-4-6-17(18)13-21(23)28)26(34)30-22-9-8-19(14-20(22)27)25(29)32(2)10-11-35-3/h4-9,12-15,29H,10-11H2,1-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140431
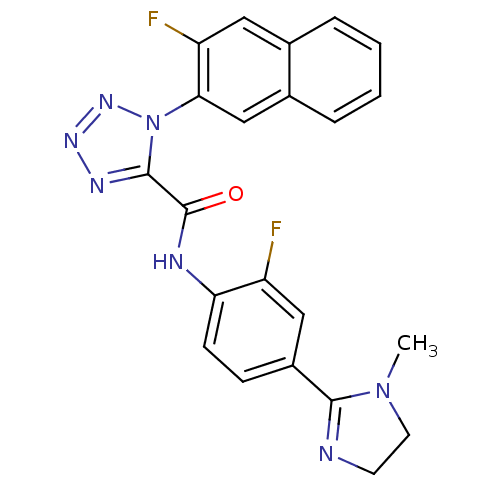
(1-(3-Fluoro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2nnnn2-c2cc3ccccc3cc2F)c(F)c1 |c:4| Show InChI InChI=1S/C22H17F2N7O/c1-30-9-8-25-20(30)15-6-7-18(16(23)11-15)26-22(32)21-27-28-29-31(21)19-12-14-5-3-2-4-13(14)10-17(19)24/h2-7,10-12H,8-9H2,1H3,(H,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140412
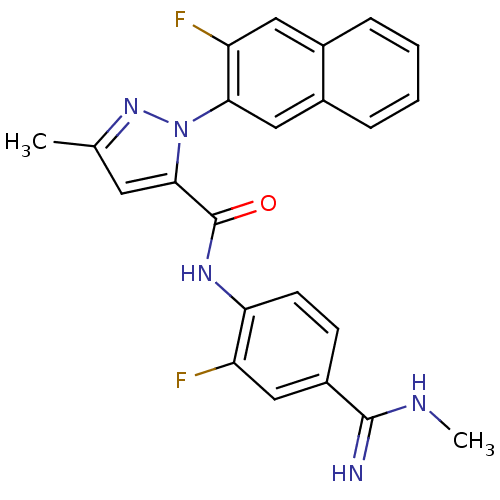
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CNC(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C23H19F2N5O/c1-13-9-21(23(31)28-19-8-7-16(11-17(19)24)22(26)27-2)30(29-13)20-12-15-6-4-3-5-14(15)10-18(20)25/h3-12H,1-2H3,(H2,26,27)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140408

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(=N)N2CCOCC2)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C26H23F2N5O2/c1-16-12-24(33(31-16)23-15-18-5-3-2-4-17(18)13-21(23)28)26(34)30-22-7-6-19(14-20(22)27)25(29)32-8-10-35-11-9-32/h2-7,12-15,29H,8-11H2,1H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140426
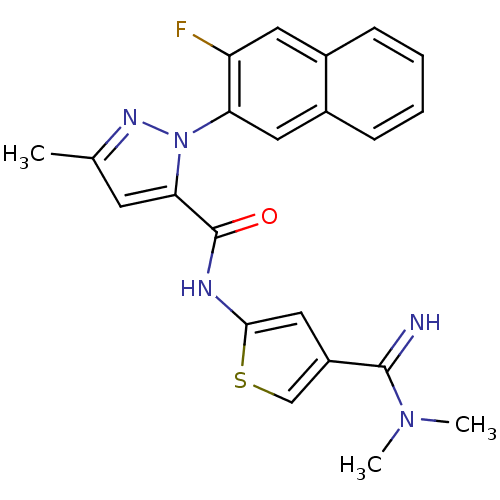
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1csc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c1 Show InChI InChI=1S/C22H20FN5OS/c1-13-8-19(22(29)25-20-11-16(12-30-20)21(24)27(2)3)28(26-13)18-10-15-7-5-4-6-14(15)9-17(18)23/h4-12,24H,1-3H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 377 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140440
(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1cccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c1 Show InChI InChI=1S/C24H22FN5O/c1-15-11-22(24(31)27-19-10-6-9-18(12-19)23(26)29(2)3)30(28-15)21-14-17-8-5-4-7-16(17)13-20(21)25/h4-14,26H,1-3H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140436

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)s1 Show InChI InChI=1S/C22H20FN5OS/c1-13-10-18(22(29)25-20-9-8-19(30-20)21(24)27(2)3)28(26-13)17-12-15-7-5-4-6-14(15)11-16(17)23/h4-12,24H,1-3H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 511 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140419

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)C(N)=N)n(n1)-c1cc2ccccc2cc1F Show InChI InChI=1S/C22H17F2N5O/c1-12-8-20(22(30)27-18-7-6-15(21(25)26)10-16(18)23)29(28-12)19-11-14-5-3-2-4-13(14)9-17(19)24/h2-11H,1H3,(H3,25,26)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 777 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50140413

(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against trypsin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50140413

(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against tissue type plasminogen activator |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Rattus norvegicus) | BDBM50140424

(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against plasmin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50140424

(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against trypsin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50140424

(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against activated protein C was determined |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Rattus norvegicus) | BDBM50140407

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C24H21F2N5O/c1-14-10-22(24(32)28-20-9-8-17(12-18(20)25)23(27)30(2)3)31(29-14)21-13-16-7-5-4-6-15(16)11-19(21)26/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against plasmin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM50140424

(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against kallikrein |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50140407

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C24H21F2N5O/c1-14-10-22(24(32)28-20-9-8-17(12-18(20)25)23(27)30(2)3)31(29-14)21-13-16-7-5-4-6-15(16)11-19(21)26/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against tissue type plasminogen activator |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50140416

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 |c:4| Show InChI InChI=1S/C25H21F2N5O/c1-15-11-23(32(30-15)22-14-17-6-4-3-5-16(17)12-20(22)27)25(33)29-21-8-7-18(13-19(21)26)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against thrombin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50140416

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 |c:4| Show InChI InChI=1S/C25H21F2N5O/c1-15-11-23(32(30-15)22-14-17-6-4-3-5-16(17)12-20(22)27)25(33)29-21-8-7-18(13-19(21)26)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against trypsin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50140416

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 |c:4| Show InChI InChI=1S/C25H21F2N5O/c1-15-11-23(32(30-15)22-14-17-6-4-3-5-16(17)12-20(22)27)25(33)29-21-8-7-18(13-19(21)26)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against tissue type plasminogen activator |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM50140413

(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against kallikrein |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM50140407

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C24H21F2N5O/c1-14-10-22(24(32)28-20-9-8-17(12-18(20)25)23(27)30(2)3)31(29-14)21-13-16-7-5-4-6-15(16)11-19(21)26/h4-13,27H,1-3H3,(H,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against kallikrein |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50140413

(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against thrombin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50140416

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 |c:4| Show InChI InChI=1S/C25H21F2N5O/c1-15-11-23(32(30-15)22-14-17-6-4-3-5-16(17)12-20(22)27)25(33)29-21-8-7-18(13-19(21)26)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against activated protein C was determined |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50140424

(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against tissue type plasminogen activator |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50140424

(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against thrombin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50140413

(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against activated protein C was determined |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50140407

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C24H21F2N5O/c1-14-10-22(24(32)28-20-9-8-17(12-18(20)25)23(27)30(2)3)31(29-14)21-13-16-7-5-4-6-15(16)11-19(21)26/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against trypsin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Rattus norvegicus) | BDBM50140413

(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against plasmin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM50140416

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 |c:4| Show InChI InChI=1S/C25H21F2N5O/c1-15-11-23(32(30-15)22-14-17-6-4-3-5-16(17)12-20(22)27)25(33)29-21-8-7-18(13-19(21)26)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against kallikrein |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Rattus norvegicus) | BDBM50140416

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 |c:4| Show InChI InChI=1S/C25H21F2N5O/c1-15-11-23(32(30-15)22-14-17-6-4-3-5-16(17)12-20(22)27)25(33)29-21-8-7-18(13-19(21)26)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against plasmin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50140407

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C24H21F2N5O/c1-14-10-22(24(32)28-20-9-8-17(12-18(20)25)23(27)30(2)3)31(29-14)21-13-16-7-5-4-6-15(16)11-19(21)26/h4-13,27H,1-3H3,(H,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against thrombin |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50140407

(2-(3-Fluoro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2cc3ccccc3cc2F)c(F)c1 Show InChI InChI=1S/C24H21F2N5O/c1-14-10-22(24(32)28-20-9-8-17(12-18(20)25)23(27)30(2)3)31(29-14)21-13-16-7-5-4-6-15(16)11-19(21)26/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against activated protein C was determined |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data