 Found 72 hits of Enzyme Inhibition Constant Data
Found 72 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144092
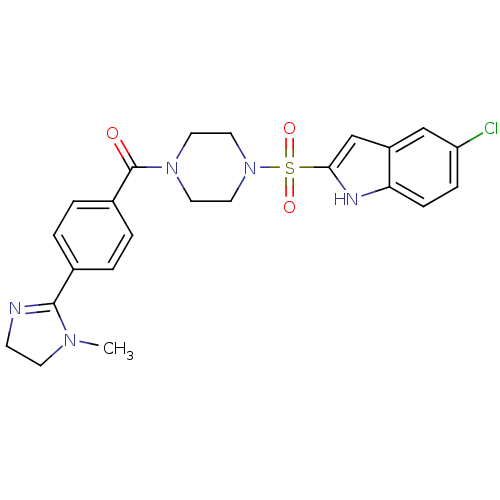
(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144106
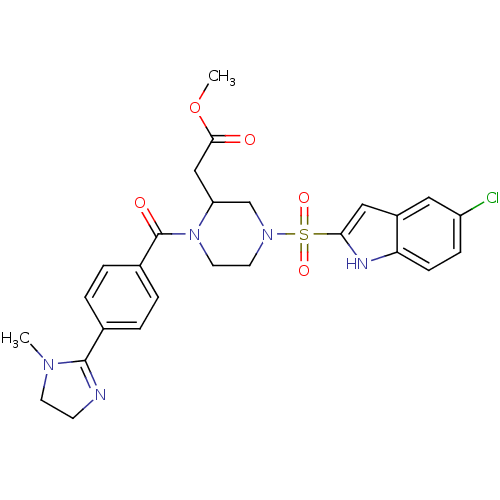
(CHEMBL65146 | {4-(5-Chloro-1H-indole-2-sulfonyl)-1...)Show SMILES COC(=O)CC1CN(CCN1C(=O)c1ccc(cc1)C1=NCCN1C)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |t:21| Show InChI InChI=1S/C26H28ClN5O5S/c1-30-10-9-28-25(30)17-3-5-18(6-4-17)26(34)32-12-11-31(16-21(32)15-24(33)37-2)38(35,36)23-14-19-13-20(27)7-8-22(19)29-23/h3-8,13-14,21,29H,9-12,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50144143

(2-{4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-[4-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCOCC1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:4| Show InChI InChI=1S/C29H32ClN5O5S2/c1-32-9-8-31-28(32)20-2-4-21(5-3-20)29(37)35-11-10-34(19-24(35)18-26(36)33-12-14-40-15-13-33)42(38,39)27-16-22-6-7-23(30)17-25(22)41-27/h2-7,16-17,24H,8-15,18-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against thrombin was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144145

(1-Azetidin-1-yl-2-{4-(5-chloro-1H-indole-2-sulfony...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C28H31ClN6O4S/c1-32-12-9-30-27(32)19-3-5-20(6-4-19)28(37)35-14-13-34(18-23(35)17-26(36)33-10-2-11-33)40(38,39)25-16-21-15-22(29)7-8-24(21)31-25/h3-8,15-16,23,31H,2,9-14,17-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144092

(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144142

(2-{4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-[4-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCCC1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:4| Show InChI InChI=1S/C29H32ClN5O4S2/c1-32-13-10-31-28(32)20-4-6-21(7-5-20)29(37)35-15-14-34(19-24(35)18-26(36)33-11-2-3-12-33)41(38,39)27-16-22-8-9-23(30)17-25(22)40-27/h4-9,16-17,24H,2-3,10-15,18-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144096
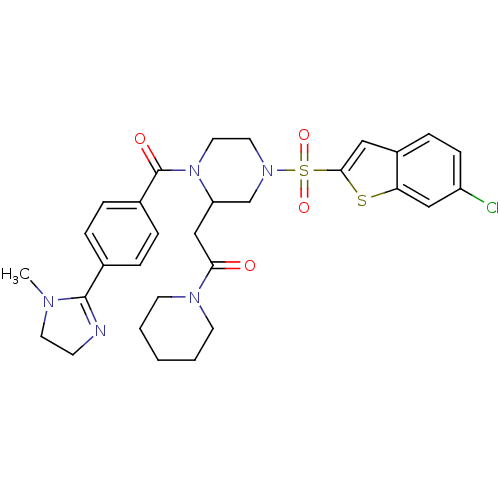
(2-{4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-[4-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCCCC1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:4| Show InChI InChI=1S/C30H34ClN5O4S2/c1-33-14-11-32-29(33)21-5-7-22(8-6-21)30(38)36-16-15-35(20-25(36)19-27(37)34-12-3-2-4-13-34)42(39,40)28-17-23-9-10-24(31)18-26(23)41-28/h5-10,17-18,25H,2-4,11-16,19-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144128

(2-{4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methy...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCCCC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C30H35ClN6O4S/c1-34-14-11-32-29(34)21-5-7-22(8-6-21)30(39)37-16-15-36(20-25(37)19-28(38)35-12-3-2-4-13-35)42(40,41)27-18-23-17-24(31)9-10-26(23)33-27/h5-10,17-18,25,33H,2-4,11-16,19-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144099
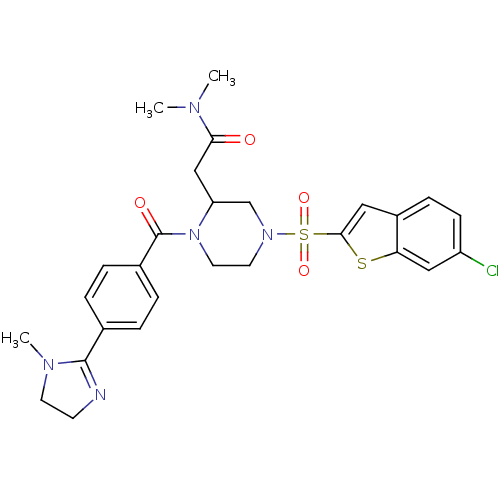
(2-{4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-[4-...)Show SMILES CN(C)C(=O)CC1CN(CCN1C(=O)c1ccc(cc1)C1=NCCN1C)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |t:22| Show InChI InChI=1S/C27H30ClN5O4S2/c1-30(2)24(34)16-22-17-32(39(36,37)25-14-20-8-9-21(28)15-23(20)38-25)12-13-33(22)27(35)19-6-4-18(5-7-19)26-29-10-11-31(26)3/h4-9,14-15,22H,10-13,16-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144112
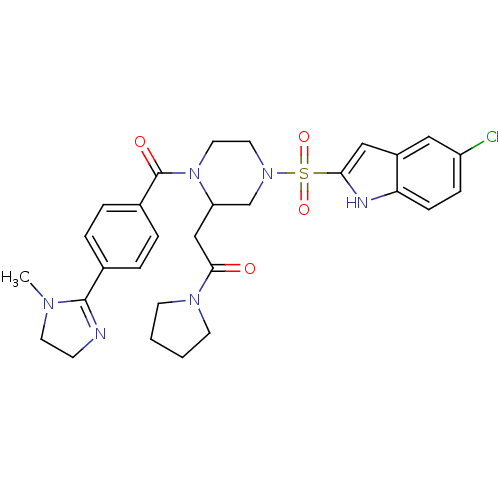
(2-{4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methy...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCCC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C29H33ClN6O4S/c1-33-13-10-31-28(33)20-4-6-21(7-5-20)29(38)36-15-14-35(19-24(36)18-27(37)34-11-2-3-12-34)41(39,40)26-17-22-16-23(30)8-9-25(22)32-26/h4-9,16-17,24,32H,2-3,10-15,18-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144136

(2-{4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methy...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCN(CC1)c1ccncc1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C34H37ClN8O4S/c1-39-13-12-37-33(39)24-2-4-25(5-3-24)34(45)43-19-18-42(48(46,47)31-21-26-20-27(35)6-7-30(26)38-31)23-29(43)22-32(44)41-16-14-40(15-17-41)28-8-10-36-11-9-28/h2-11,20-21,29,38H,12-19,22-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144126
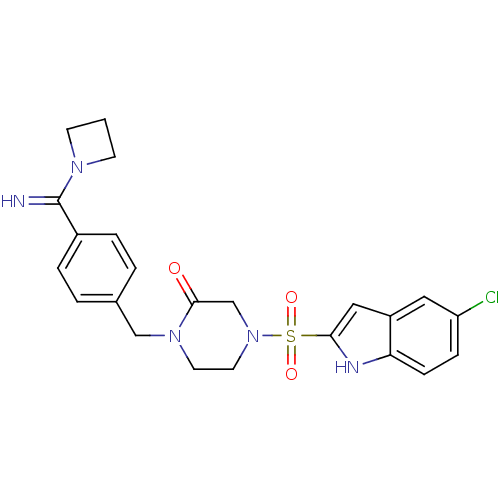
(1-[4-(Azetidin-1-yl-imino-methyl)-benzyl]-4-(5-chl...)Show SMILES Clc1ccc2[nH]c(cc2c1)S(=O)(=O)N1CCN(Cc2ccc(cc2)C(=N)N2CCC2)C(=O)C1 Show InChI InChI=1S/C23H24ClN5O3S/c24-19-6-7-20-18(12-19)13-21(26-20)33(31,32)29-11-10-28(22(30)15-29)14-16-2-4-17(5-3-16)23(25)27-8-1-9-27/h2-7,12-13,25-26H,1,8-11,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144127

(2-{4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-[4-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCN(CC1)c1ccncc1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:4| Show InChI InChI=1S/C34H36ClN7O4S2/c1-38-13-12-37-33(38)24-2-4-25(5-3-24)34(44)42-19-18-41(48(45,46)32-20-26-6-7-27(35)21-30(26)47-32)23-29(42)22-31(43)40-16-14-39(15-17-40)28-8-10-36-11-9-28/h2-11,20-21,29H,12-19,22-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144135
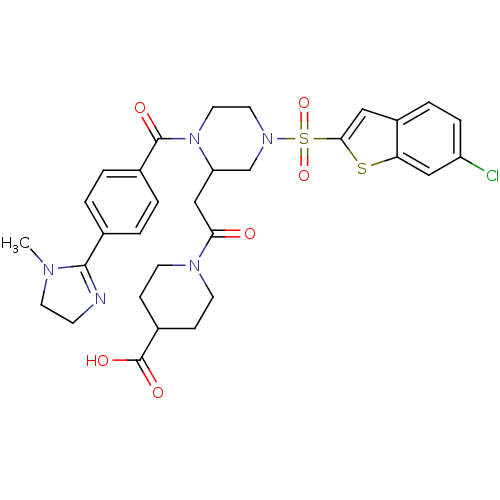
(1-(2-{4-(6-chloro-benzo[b]thiophene-2-sulfonyl)-1-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCC(CC1)C(O)=O)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:4| Show InChI InChI=1S/C31H34ClN5O6S2/c1-34-13-10-33-29(34)20-2-4-21(5-3-20)30(39)37-15-14-36(45(42,43)28-16-23-6-7-24(32)17-26(23)44-28)19-25(37)18-27(38)35-11-8-22(9-12-35)31(40)41/h2-7,16-17,22,25H,8-15,18-19H2,1H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144114
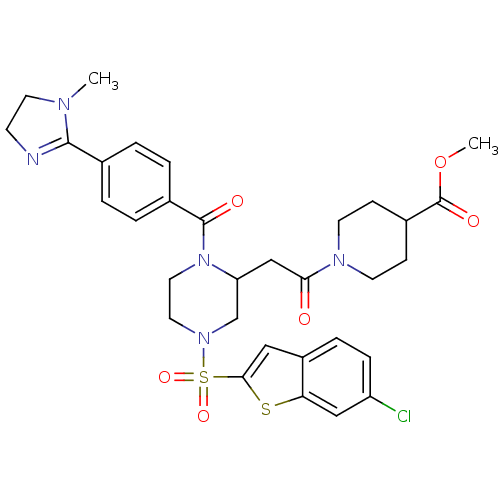
(1-(2-{4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-...)Show SMILES COC(=O)C1CCN(CC1)C(=O)CC1CN(CCN1C(=O)c1ccc(cc1)C1=NCCN1C)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |t:30| Show InChI InChI=1S/C32H36ClN5O6S2/c1-35-14-11-34-30(35)21-3-5-22(6-4-21)31(40)38-16-15-37(46(42,43)29-17-24-7-8-25(33)18-27(24)45-29)20-26(38)19-28(39)36-12-9-23(10-13-36)32(41)44-2/h3-8,17-18,23,26H,9-16,19-20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144124
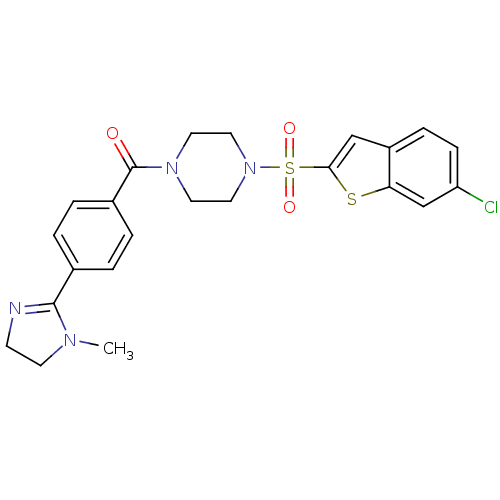
(CHEMBL293462 | [4-(6-Chloro-benzo[b]thiophene-2-su...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:4| Show InChI InChI=1S/C23H23ClN4O3S2/c1-26-9-8-25-22(26)16-2-4-17(5-3-16)23(29)27-10-12-28(13-11-27)33(30,31)21-14-18-6-7-19(24)15-20(18)32-21/h2-7,14-15H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50144124

(CHEMBL293462 | [4-(6-Chloro-benzo[b]thiophene-2-su...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:4| Show InChI InChI=1S/C23H23ClN4O3S2/c1-26-9-8-25-22(26)16-2-4-17(5-3-16)23(29)27-10-12-28(13-11-27)33(30,31)21-14-18-6-7-19(24)15-20(18)32-21/h2-7,14-15H,8-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against thrombin was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144138

(1-Azetidin-1-yl-2-{4-(6-chloro-benzo[b]thiophene-2...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCC1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:4| Show InChI InChI=1S/C28H30ClN5O4S2/c1-31-12-9-30-27(31)19-3-5-20(6-4-19)28(36)34-14-13-33(18-23(34)17-25(35)32-10-2-11-32)40(37,38)26-15-21-7-8-22(29)16-24(21)39-26/h3-8,15-16,23H,2,9-14,17-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144132

(2-{4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methy...)Show SMILES CN(C)C(=O)CC1CN(CCN1C(=O)c1ccc(cc1)C1=NCCN1C)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |t:22| Show InChI InChI=1S/C27H31ClN6O4S/c1-31(2)25(35)16-22-17-33(39(37,38)24-15-20-14-21(28)8-9-23(20)30-24)12-13-34(22)27(36)19-6-4-18(5-7-19)26-29-10-11-32(26)3/h4-9,14-15,22,30H,10-13,16-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144111

(2-{4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methy...)Show SMILES CNC(=O)CC1CN(CCN1C(=O)c1ccc(cc1)C1=NCCN1C)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |t:21| Show InChI InChI=1S/C26H29ClN6O4S/c1-28-23(34)15-21-16-32(38(36,37)24-14-19-13-20(27)7-8-22(19)30-24)11-12-33(21)26(35)18-5-3-17(4-6-18)25-29-9-10-31(25)2/h3-8,13-14,21,30H,9-12,15-16H2,1-2H3,(H,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144113

(1-[4-(Azetidin-1-yl-imino-methyl)-benzyl]-4-(6-chl...)Show SMILES Clc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(Cc2ccc(cc2)C(=N)N2CCC2)C(=O)C1 Show InChI InChI=1S/C23H23ClN4O3S2/c24-19-7-6-18-12-22(32-20(18)13-19)33(30,31)28-11-10-27(21(29)15-28)14-16-2-4-17(5-3-16)23(25)26-8-1-9-26/h2-7,12-13,25H,1,8-11,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144084
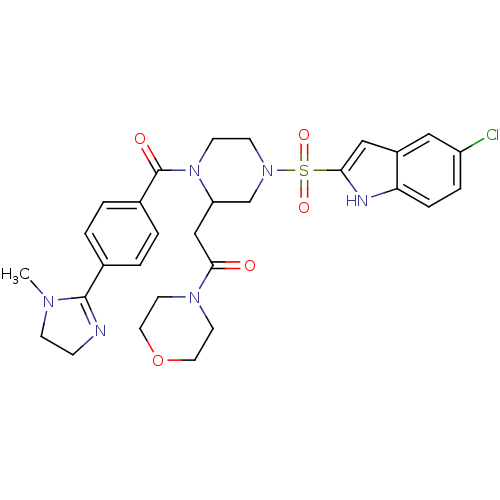
(2-{4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methy...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCOCC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C29H33ClN6O5S/c1-33-9-8-31-28(33)20-2-4-21(5-3-20)29(38)36-11-10-35(19-24(36)18-27(37)34-12-14-41-15-13-34)42(39,40)26-17-22-16-23(30)6-7-25(22)32-26/h2-7,16-17,24,32H,8-15,18-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144133

(CHEMBL65816 | [4-(5-Chloro-1H-indole-2-sulfonyl)-p...)Show SMILES CCN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:5| Show InChI InChI=1S/C24H26ClN5O3S/c1-2-28-10-9-26-23(28)17-3-5-18(6-4-17)24(31)29-11-13-30(14-12-29)34(32,33)22-16-19-15-20(25)7-8-21(19)27-22/h3-8,15-16,27H,2,9-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144118

(4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methyl-4...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1C(O)=O)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C24H24ClN5O5S/c1-28-9-8-26-22(28)15-2-4-16(5-3-15)23(31)30-11-10-29(14-20(30)24(32)33)36(34,35)21-13-17-12-18(25)6-7-19(17)27-21/h2-7,12-13,20,27H,8-11,14H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144115
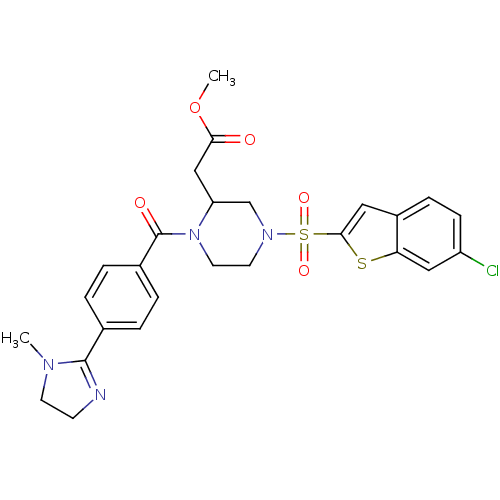
(CHEMBL63307 | {4-(6-Chloro-benzo[b]thiophene-2-sul...)Show SMILES COC(=O)CC1CN(CCN1C(=O)c1ccc(cc1)C1=NCCN1C)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |t:21| Show InChI InChI=1S/C26H27ClN4O5S2/c1-29-10-9-28-25(29)17-3-5-18(6-4-17)26(33)31-12-11-30(16-21(31)15-23(32)36-2)38(34,35)24-13-19-7-8-20(27)14-22(19)37-24/h3-8,13-14,21H,9-12,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144097
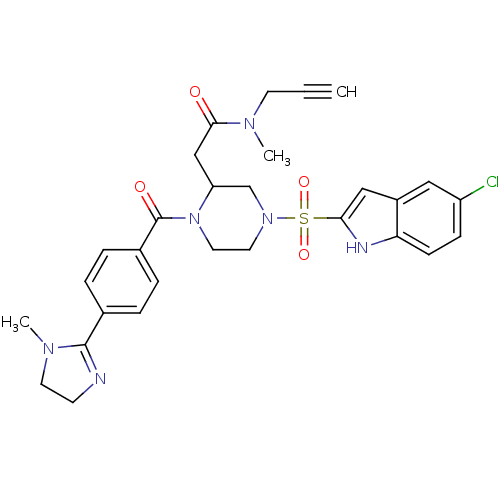
(2-{4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methy...)Show SMILES CN(CC#C)C(=O)CC1CN(CCN1C(=O)c1ccc(cc1)C1=NCCN1C)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |t:24| Show InChI InChI=1S/C29H31ClN6O4S/c1-4-12-33(2)27(37)18-24-19-35(41(39,40)26-17-22-16-23(30)9-10-25(22)32-26)14-15-36(24)29(38)21-7-5-20(6-8-21)28-31-11-13-34(28)3/h1,5-10,16-17,24,32H,11-15,18-19H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144143

(2-{4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-[4-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(=O)N1CCOCC1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:4| Show InChI InChI=1S/C29H32ClN5O5S2/c1-32-9-8-31-28(32)20-2-4-21(5-3-20)29(37)35-11-10-34(19-24(35)18-26(36)33-12-14-40-15-13-33)42(38,39)27-16-22-6-7-23(30)17-25(22)41-27/h2-7,16-17,24H,8-15,18-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144083
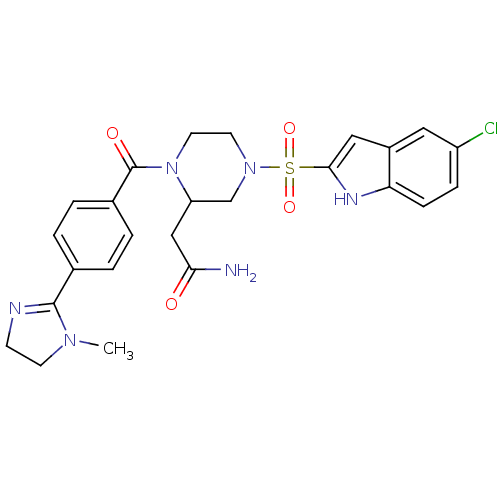
(2-{4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methy...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(N)=O)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C25H27ClN6O4S/c1-30-9-8-28-24(30)16-2-4-17(5-3-16)25(34)32-11-10-31(15-20(32)14-22(27)33)37(35,36)23-13-18-12-19(26)6-7-21(18)29-23/h2-7,12-13,20,29H,8-11,14-15H2,1H3,(H2,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144119

(2-{4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-[4-...)Show SMILES CN(CC#C)C(=O)CC1CN(CCN1C(=O)c1ccc(cc1)C1=NCCN1C)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |t:24| Show InChI InChI=1S/C29H30ClN5O4S2/c1-4-12-32(2)26(36)18-24-19-34(41(38,39)27-16-22-9-10-23(30)17-25(22)40-27)14-15-35(24)29(37)21-7-5-20(6-8-21)28-31-11-13-33(28)3/h1,5-10,16-17,24H,11-15,18-19H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144104

(4-[4-(5-Chloro-1H-indole-2-sulfonyl)-piperazine-1-...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C22H24ClN5O3S/c1-26(2)21(24)15-3-5-16(6-4-15)22(29)27-9-11-28(12-10-27)32(30,31)20-14-17-13-18(23)7-8-19(17)25-20/h3-8,13-14,24-25H,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144103
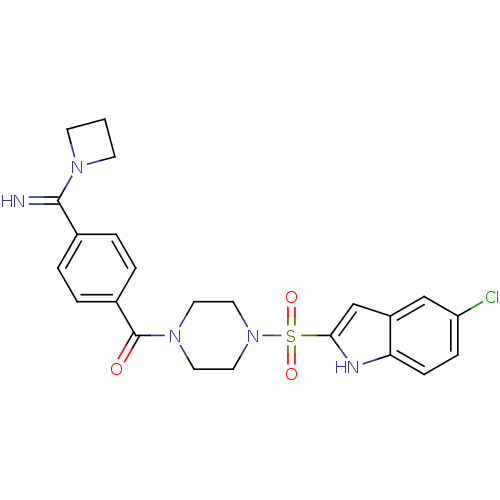
(CHEMBL417738 | [4-(Azetidin-1-yl-imino-methyl)-phe...)Show SMILES Clc1ccc2[nH]c(cc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C(=N)N1CCC1 Show InChI InChI=1S/C23H24ClN5O3S/c24-19-6-7-20-18(14-19)15-21(26-20)33(31,32)29-12-10-28(11-13-29)23(30)17-4-2-16(3-5-17)22(25)27-8-1-9-27/h2-7,14-15,25-26H,1,8-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144130

(CHEMBL65128 | [4-(Azetidin-1-yl-imino-methyl)-phen...)Show SMILES Clc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C(=N)N1CCC1 Show InChI InChI=1S/C23H23ClN4O3S2/c24-19-7-6-18-14-21(32-20(18)15-19)33(30,31)28-12-10-27(11-13-28)23(29)17-4-2-16(3-5-17)22(25)26-8-1-9-26/h2-7,14-15,25H,1,8-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144131
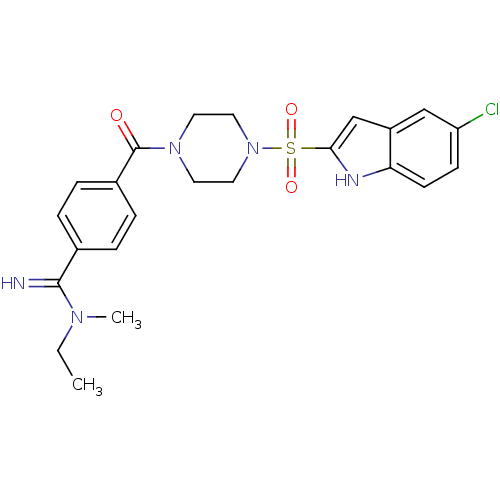
(4-[4-(5-Chloro-1H-indole-2-sulfonyl)-piperazine-1-...)Show SMILES CCN(C)C(=N)c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C23H26ClN5O3S/c1-3-27(2)22(25)16-4-6-17(7-5-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)8-9-20(18)26-21/h4-9,14-15,25-26H,3,10-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144098
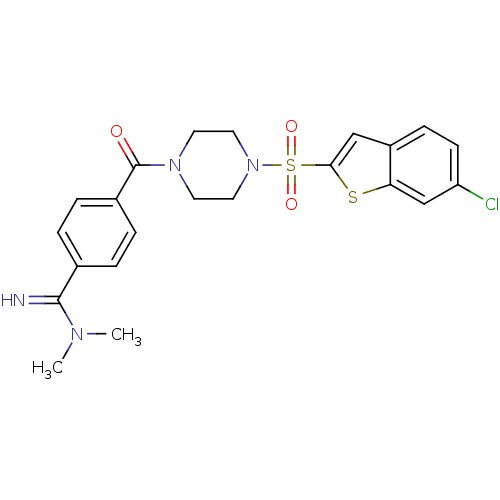
(4-[4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-piper...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25(2)21(24)15-3-5-16(6-4-15)22(28)26-9-11-27(12-10-26)32(29,30)20-13-17-7-8-18(23)14-19(17)31-20/h3-8,13-14,24H,9-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144088

(4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methyl-4...)Show SMILES COC(=O)C1CN(CCN1C(=O)c1ccc(cc1)C1=NCCN1C)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |t:20| Show InChI InChI=1S/C25H26ClN5O5S/c1-29-10-9-27-23(29)16-3-5-17(6-4-16)24(32)31-12-11-30(15-21(31)25(33)36-2)37(34,35)22-14-18-13-19(26)7-8-20(18)28-22/h3-8,13-14,21,28H,9-12,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144117

(CHEMBL291430 | {4-(6-Chloro-benzo[b]thiophene-2-su...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(O)=O)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:4| Show InChI InChI=1S/C25H25ClN4O5S2/c1-28-9-8-27-24(28)16-2-4-17(5-3-16)25(33)30-11-10-29(15-20(30)14-22(31)32)37(34,35)23-12-18-6-7-19(26)13-21(18)36-23/h2-7,12-13,20H,8-11,14-15H2,1H3,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144123
(4-[4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-2-oxo...)Show SMILES CN(C)C(=N)c1ccc(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc1 Show InChI InChI=1S/C22H23ClN4O3S2/c1-25(2)22(24)16-5-3-15(4-6-16)13-26-9-10-27(14-20(26)28)32(29,30)21-11-17-7-8-18(23)12-19(17)31-21/h3-8,11-12,24H,9-10,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144109
(CHEMBL66535 | [4-(6-Bromo-naphthalene-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Br)ccc2c1 |c:4| Show InChI InChI=1S/C25H25BrN4O3S/c1-28-11-10-27-24(28)18-2-4-19(5-3-18)25(31)29-12-14-30(15-13-29)34(32,33)23-9-7-20-16-22(26)8-6-21(20)17-23/h2-9,16-17H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144085
(CHEMBL436356 | [4-(6-Chloro-benzo[b]thiophene-2-su...)Show SMILES CCN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 |c:5| Show InChI InChI=1S/C24H25ClN4O3S2/c1-2-27-10-9-26-23(27)17-3-5-18(6-4-17)24(30)28-11-13-29(14-12-28)34(31,32)22-15-19-7-8-20(25)16-21(19)33-22/h3-8,15-16H,2,9-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144087

(4-[4-(5-Chloro-1H-indole-2-sulfonyl)-2-oxo-piperaz...)Show SMILES CN(C)C(=N)c1ccc(CN2CCN(CC2=O)S(=O)(=O)c2cc3cc(Cl)ccc3[nH]2)cc1 Show InChI InChI=1S/C22H24ClN5O3S/c1-26(2)22(24)16-5-3-15(4-6-16)13-27-9-10-28(14-21(27)29)32(30,31)20-12-17-11-18(23)7-8-19(17)25-20/h3-8,11-12,24-25H,9-10,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144116

(CHEMBL292941 | {4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1CC(O)=O)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C25H26ClN5O5S/c1-29-9-8-27-24(29)16-2-4-17(5-3-16)25(34)31-11-10-30(15-20(31)14-23(32)33)37(35,36)22-13-18-12-19(26)6-7-21(18)28-22/h2-7,12-13,20,28H,8-11,14-15H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144108

(4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-[4-(1-...)Show SMILES CN1CCN=C1c1ccc(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc1 |c:4| Show InChI InChI=1S/C23H23ClN4O3S2/c1-26-9-8-25-23(26)17-4-2-16(3-5-17)14-27-10-11-28(15-21(27)29)33(30,31)22-12-18-6-7-19(24)13-20(18)32-22/h2-7,12-13H,8-11,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144140
(4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(1-methyl-4...)Show SMILES CN1CCN=C1c1ccc(CN2CCN(CC2=O)S(=O)(=O)c2cc3cc(Cl)ccc3[nH]2)cc1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-23(27)17-4-2-16(3-5-17)14-28-10-11-29(15-22(28)30)33(31,32)21-13-18-12-19(24)6-7-20(18)26-21/h2-7,12-13,26H,8-11,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144101
(4-[4-(6-Bromo-naphthalene-2-sulfonyl)-piperazine-1...)Show SMILES NC(=N)c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Br)ccc2c1 Show InChI InChI=1S/C22H21BrN4O3S/c23-19-7-5-18-14-20(8-6-17(18)13-19)31(29,30)27-11-9-26(10-12-27)22(28)16-3-1-15(2-4-16)21(24)25/h1-8,13-14H,9-12H2,(H3,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144100
(CHEMBL62758 | [4-(6-Bromo-naphthalene-2-sulfonyl)-...)Show SMILES CCN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Br)ccc2c1 |c:5| Show InChI InChI=1S/C26H27BrN4O3S/c1-2-29-12-11-28-25(29)19-3-5-20(6-4-19)26(32)30-13-15-31(16-14-30)35(33,34)24-10-8-21-17-23(27)9-7-22(21)18-24/h3-10,17-18H,2,11-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144121
(CHEMBL66362 | [4-(6-Chloro-benzo[b]thiophene-2-sul...)Show SMILES Clc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C(=N)N1CCCC1 Show InChI InChI=1S/C24H25ClN4O3S2/c25-20-8-7-19-15-22(33-21(19)16-20)34(31,32)29-13-11-28(12-14-29)24(30)18-5-3-17(4-6-18)23(26)27-9-1-2-10-27/h3-8,15-16,26H,1-2,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144122
(CHEMBL304859 | [4-(6-Chloro-benzo[b]thiophene-2-su...)Show SMILES Clc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C(=N)N1CCCCC1 Show InChI InChI=1S/C25H27ClN4O3S2/c26-21-9-8-20-16-23(34-22(20)17-21)35(32,33)30-14-12-29(13-15-30)25(31)19-6-4-18(5-7-19)24(27)28-10-2-1-3-11-28/h4-9,16-17,27H,1-3,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144137
(CHEMBL292254 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES Clc1ccc2[nH]c(cc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C(=N)N1CCCC1 Show InChI InChI=1S/C24H26ClN5O3S/c25-20-7-8-21-19(15-20)16-22(27-21)34(32,33)30-13-11-29(12-14-30)24(31)18-5-3-17(4-6-18)23(26)28-9-1-2-10-28/h3-8,15-16,26-27H,1-2,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144144

(4-[4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-piper...)Show SMILES CCN(C)C(=N)c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2ccc(Cl)cc2s1 Show InChI InChI=1S/C23H25ClN4O3S2/c1-3-26(2)22(25)16-4-6-17(7-5-16)23(29)27-10-12-28(13-11-27)33(30,31)21-14-18-8-9-19(24)15-20(18)32-21/h4-9,14-15,25H,3,10-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144089

(4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-[4-(im...)Show SMILES Clc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(Cc2ccc(cc2)C(=N)N2CCCCC2)C(=O)C1 Show InChI InChI=1S/C25H27ClN4O3S2/c26-21-9-8-20-14-24(34-22(20)15-21)35(32,33)30-13-12-29(23(31)17-30)16-18-4-6-19(7-5-18)25(27)28-10-2-1-3-11-28/h4-9,14-15,27H,1-3,10-13,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144094
(CHEMBL65613 | [4-(5-Chloro-1H-indole-2-sulfonyl)-p...)Show SMILES Clc1ccc2[nH]c(cc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C(=N)N1CCCCC1 Show InChI InChI=1S/C25H28ClN5O3S/c26-21-8-9-22-20(16-21)17-23(28-22)35(33,34)31-14-12-30(13-15-31)25(32)19-6-4-18(5-7-19)24(27)29-10-2-1-3-11-29/h4-9,16-17,27-28H,1-3,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144105
(4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-[4-(im...)Show SMILES Clc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(Cc2ccc(cc2)C(=N)N2CCCC2)C(=O)C1 Show InChI InChI=1S/C24H25ClN4O3S2/c25-20-8-7-19-13-23(33-21(19)14-20)34(31,32)29-12-11-28(22(30)16-29)15-17-3-5-18(6-4-17)24(26)27-9-1-2-10-27/h3-8,13-14,26H,1-2,9-12,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144139

(4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(imino-pipe...)Show SMILES Clc1ccc2[nH]c(cc2c1)S(=O)(=O)N1CCN(Cc2ccc(cc2)C(=N)N2CCCCC2)C(=O)C1 Show InChI InChI=1S/C25H28ClN5O3S/c26-21-8-9-22-20(14-21)15-23(28-22)35(33,34)31-13-12-30(24(32)17-31)16-18-4-6-19(7-5-18)25(27)29-10-2-1-3-11-29/h4-9,14-15,27-28H,1-3,10-13,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144102
(CHEMBL302178 | [4-(6-Bromo-naphthalene-2-sulfonyl)...)Show SMILES Brc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C(=N)N1CCCCC1 Show InChI InChI=1S/C27H29BrN4O3S/c28-24-10-8-23-19-25(11-9-22(23)18-24)36(34,35)32-16-14-31(15-17-32)27(33)21-6-4-20(5-7-21)26(29)30-12-2-1-3-13-30/h4-11,18-19,29H,1-3,12-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
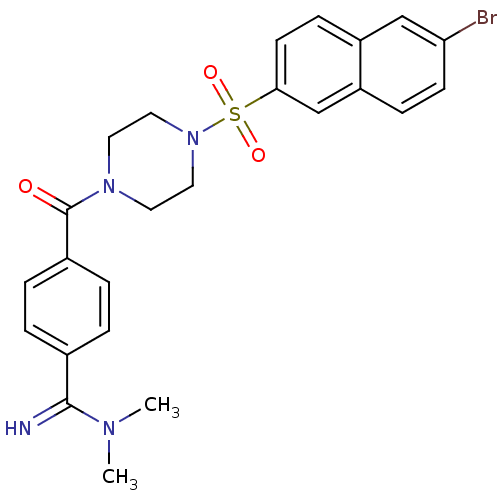
(Homo sapiens (Human)) | BDBM50144134
(4-[4-(6-Bromo-naphthalene-2-sulfonyl)-piperazine-1...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Br)ccc2c1 Show InChI InChI=1S/C24H25BrN4O3S/c1-27(2)23(26)17-3-5-18(6-4-17)24(30)28-11-13-29(14-12-28)33(31,32)22-10-8-19-15-21(25)9-7-20(19)16-22/h3-10,15-16,26H,11-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
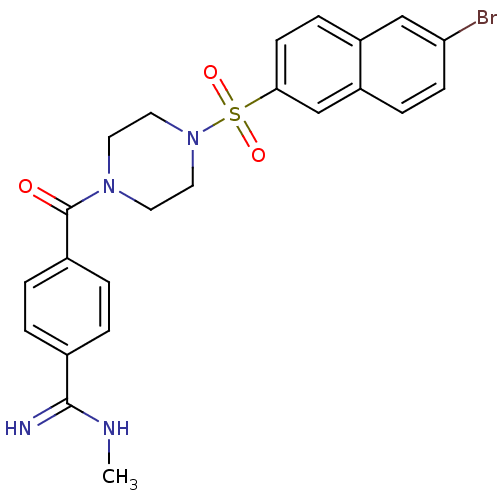
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144110
(4-[4-(6-Bromo-naphthalene-2-sulfonyl)-piperazine-1...)Show SMILES CNC(=N)c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Br)ccc2c1 Show InChI InChI=1S/C23H23BrN4O3S/c1-26-22(25)16-2-4-17(5-3-16)23(29)27-10-12-28(13-11-27)32(30,31)21-9-7-18-14-20(24)8-6-19(18)15-21/h2-9,14-15H,10-13H2,1H3,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144141

(4'-[4-(6-Bromo-naphthalene-2-sulfonyl)-piperazine-...)Show SMILES NS(=O)(=O)c1ccccc1-c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Br)ccc2c1 Show InChI InChI=1S/C27H24BrN3O5S2/c28-23-11-9-22-18-24(12-10-21(22)17-23)38(35,36)31-15-13-30(14-16-31)27(32)20-7-5-19(6-8-20)25-3-1-2-4-26(25)37(29,33)34/h1-12,17-18H,13-16H2,(H2,29,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
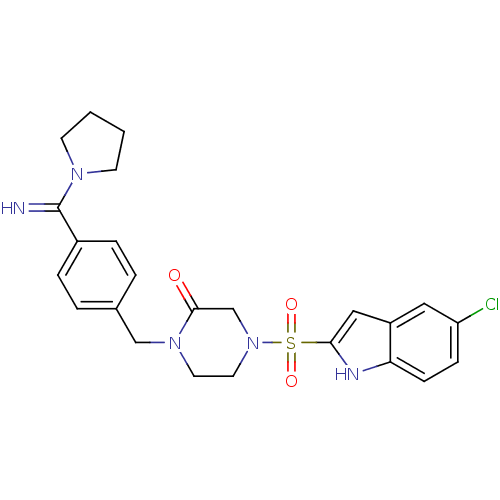
(Homo sapiens (Human)) | BDBM50144120
(4-(5-Chloro-1H-indole-2-sulfonyl)-1-[4-(imino-pyrr...)Show SMILES Clc1ccc2[nH]c(cc2c1)S(=O)(=O)N1CCN(Cc2ccc(cc2)C(=N)N2CCCC2)C(=O)C1 Show InChI InChI=1S/C24H26ClN5O3S/c25-20-7-8-21-19(13-20)14-22(27-21)34(32,33)30-12-11-29(23(31)16-30)15-17-3-5-18(6-4-17)24(26)28-9-1-2-10-28/h3-8,13-14,26-27H,1-2,9-12,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144086

(CHEMBL293682 | [4-(5-Chloro-benzo[b]thiophene-2-su...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2s1 |c:4| Show InChI InChI=1S/C23H23ClN4O3S2/c1-26-9-8-25-22(26)16-2-4-17(5-3-16)23(29)27-10-12-28(13-11-27)33(30,31)21-15-18-14-19(24)6-7-20(18)32-21/h2-7,14-15H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144107

(CHEMBL446398 | [4-(6-Bromo-naphthalene-2-sulfonyl)...)Show SMILES Brc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C(=N)N1CCCC1 Show InChI InChI=1S/C26H27BrN4O3S/c27-23-9-7-22-18-24(10-8-21(22)17-23)35(33,34)31-15-13-30(14-16-31)26(32)20-5-3-19(4-6-20)25(28)29-11-1-2-12-29/h3-10,17-18,28H,1-2,11-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144125

(CHEMBL63690 | [4-(6-Bromo-naphthalene-2-sulfonyl)-...)Show SMILES CN1CCCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1ccc2cc(Br)ccc2c1 |c:5| Show InChI InChI=1S/C26H27BrN4O3S/c1-29-12-2-11-28-25(29)19-3-5-20(6-4-19)26(32)30-13-15-31(16-14-30)35(33,34)24-10-8-21-17-23(27)9-7-22(21)18-24/h3-10,17-18H,2,11-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144095

(CHEMBL65544 | [4-(6-Bromo-naphthalene-2-sulfonyl)-...)Show SMILES Brc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C1=NCCN1 |t:32| Show InChI InChI=1S/C24H23BrN4O3S/c25-21-7-5-20-16-22(8-6-19(20)15-21)33(31,32)29-13-11-28(12-14-29)24(30)18-3-1-17(2-4-18)23-26-9-10-27-23/h1-8,15-16H,9-14H2,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
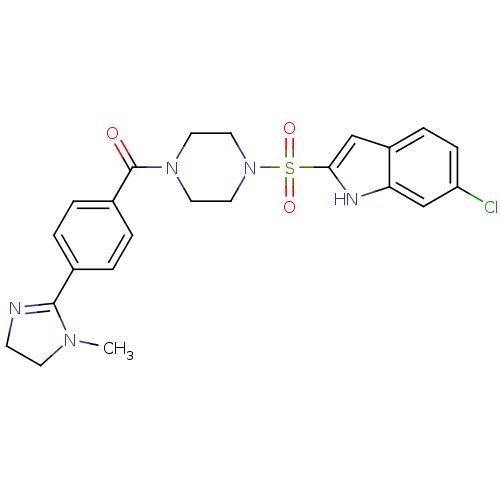
(Homo sapiens (Human)) | BDBM50144093
(CHEMBL293425 | [4-(6-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2ccc(Cl)cc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-14-18-6-7-19(24)15-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144090
(CHEMBL62563 | [4-(6-Bromo-naphthalene-2-sulfonyl)-...)Show SMILES Brc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C(=N)N1CCOCC1 Show InChI InChI=1S/C26H27BrN4O4S/c27-23-7-5-22-18-24(8-6-21(22)17-23)36(33,34)31-11-9-30(10-12-31)26(32)20-3-1-19(2-4-20)25(28)29-13-15-35-16-14-29/h1-8,17-18,28H,9-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144091

(CHEMBL65010 | [4-(6-Bromo-naphthalene-2-sulfonyl)-...)Show SMILES Brc1ccc2cc(ccc2c1)S(=O)(=O)N1CCN(CC1)C(=O)c1ccc(cc1)C1=NCCCN1 |t:32| Show InChI InChI=1S/C25H25BrN4O3S/c26-22-8-6-21-17-23(9-7-20(21)16-22)34(32,33)30-14-12-29(13-15-30)25(31)19-4-2-18(3-5-19)24-27-10-1-11-28-24/h2-9,16-17H,1,10-15H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM50144092

(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against kallikrein was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50144092

(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against thrombin was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Rattus norvegicus) | BDBM50144092

(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against plasmin was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50144092

(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against trypsin was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50144092

(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against tissue type plasminogen activator was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50144092

(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against activated protein C was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144129
(CHEMBL64947 | [4-(5-Chloro-1H-indole-2-carbonyl)-p...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)C(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C24H24ClN5O2/c1-28-9-8-26-22(28)16-2-4-17(5-3-16)23(31)29-10-12-30(13-11-29)24(32)21-15-18-14-19(25)6-7-20(18)27-21/h2-7,14-15,27H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data