 Found 47 hits of Enzyme Inhibition Constant Data
Found 47 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144342
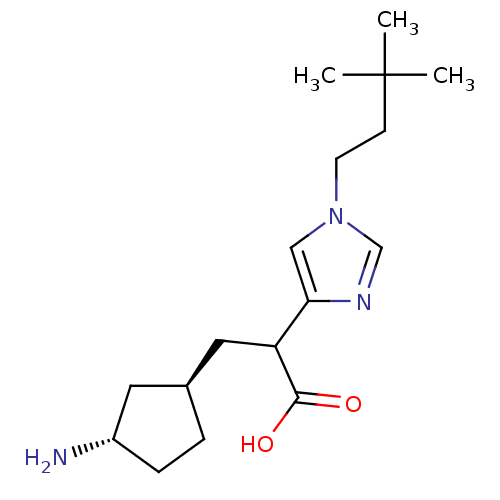
(3-((1R,3S)-3-Amino-cyclopentyl)-2-[1-(3,3-dimethyl...)Show SMILES CC(C)(C)CCn1cnc(c1)C(C[C@H]1CC[C@H](N)C1)C(O)=O Show InChI InChI=1S/C17H29N3O2/c1-17(2,3)6-7-20-10-15(19-11-20)14(16(21)22)9-12-4-5-13(18)8-12/h10-14H,4-9,18H2,1-3H3,(H,21,22)/t12-,13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144336
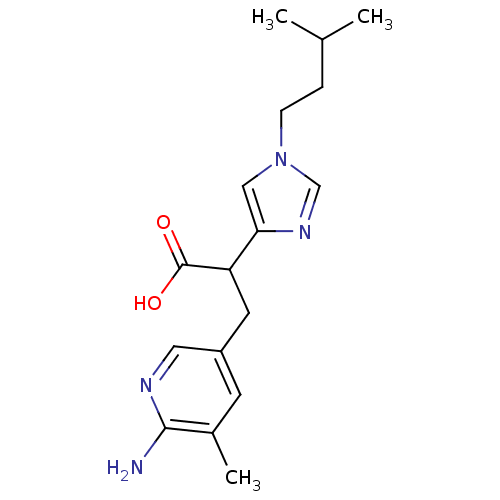
(3-(6-Amino-5-methyl-pyridin-3-yl)-2-[1-(3-methyl-b...)Show InChI InChI=1S/C17H24N4O2/c1-11(2)4-5-21-9-15(20-10-21)14(17(22)23)7-13-6-12(3)16(18)19-8-13/h6,8-11,14H,4-5,7H2,1-3H3,(H2,18,19)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144342

(3-((1R,3S)-3-Amino-cyclopentyl)-2-[1-(3,3-dimethyl...)Show SMILES CC(C)(C)CCn1cnc(c1)C(C[C@H]1CC[C@H](N)C1)C(O)=O Show InChI InChI=1S/C17H29N3O2/c1-17(2,3)6-7-20-10-15(19-11-20)14(16(21)22)9-12-4-5-13(18)8-12/h10-14H,4-9,18H2,1-3H3,(H,21,22)/t12-,13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144333
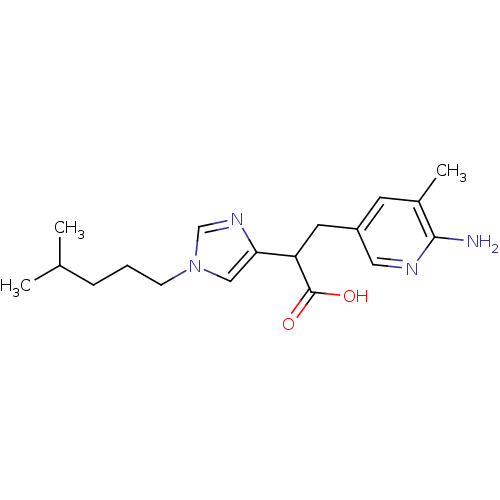
(3-(6-Amino-5-methyl-pyridin-3-yl)-2-[1-(4-methyl-p...)Show InChI InChI=1S/C18H26N4O2/c1-12(2)5-4-6-22-10-16(21-11-22)15(18(23)24)8-14-7-13(3)17(19)20-9-14/h7,9-12,15H,4-6,8H2,1-3H3,(H2,19,20)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144337
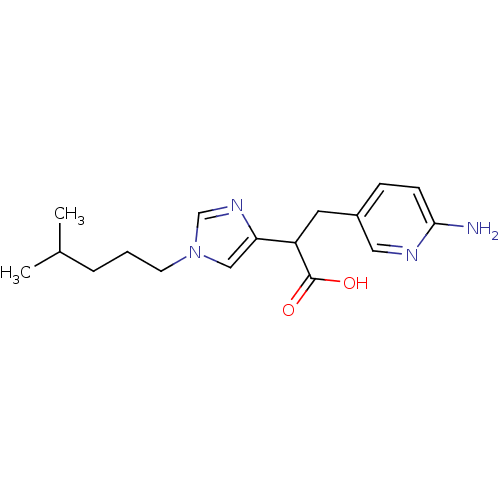
(3-(6-Amino-pyridin-3-yl)-2-[1-(4-methyl-pentyl)-1H...)Show InChI InChI=1S/C17H24N4O2/c1-12(2)4-3-7-21-10-15(20-11-21)14(17(22)23)8-13-5-6-16(18)19-9-13/h5-6,9-12,14H,3-4,7-8H2,1-2H3,(H2,18,19)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50135934
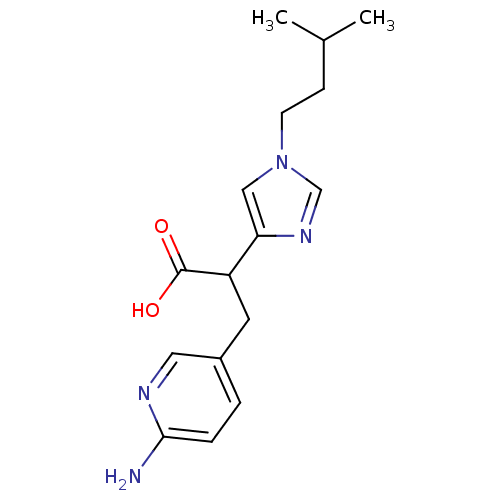
(3-(6-Amino-pyridin-3-yl)-2-[1-(3-methyl-butyl)-1H-...)Show InChI InChI=1S/C16H22N4O2/c1-11(2)5-6-20-9-14(19-10-20)13(16(21)22)7-12-3-4-15(17)18-8-12/h3-4,8-11,13H,5-7H2,1-2H3,(H2,17,18)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144337

(3-(6-Amino-pyridin-3-yl)-2-[1-(4-methyl-pentyl)-1H...)Show InChI InChI=1S/C17H24N4O2/c1-12(2)4-3-7-21-10-15(20-11-21)14(17(22)23)8-13-5-6-16(18)19-9-13/h5-6,9-12,14H,3-4,7-8H2,1-2H3,(H2,18,19)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135934

(3-(6-Amino-pyridin-3-yl)-2-[1-(3-methyl-butyl)-1H-...)Show InChI InChI=1S/C16H22N4O2/c1-11(2)5-6-20-9-14(19-10-20)13(16(21)22)7-12-3-4-15(17)18-8-12/h3-4,8-11,13H,5-7H2,1-2H3,(H2,17,18)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144333

(3-(6-Amino-5-methyl-pyridin-3-yl)-2-[1-(4-methyl-p...)Show InChI InChI=1S/C18H26N4O2/c1-12(2)5-4-6-22-10-16(21-11-22)15(18(23)24)8-14-7-13(3)17(19)20-9-14/h7,9-12,15H,4-6,8H2,1-3H3,(H2,19,20)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144316
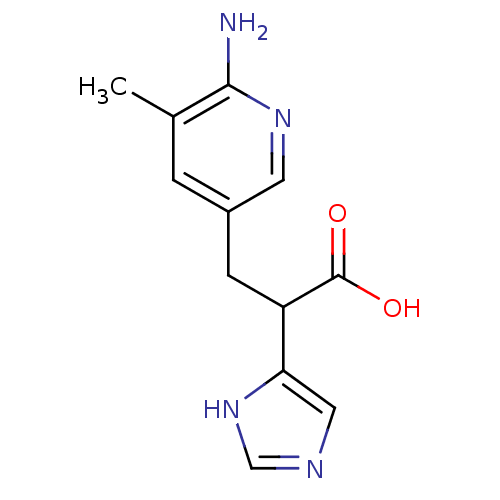
(3-(6-Amino-5-methyl-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C12H14N4O2/c1-7-2-8(4-15-11(7)13)3-9(12(17)18)10-5-14-6-16-10/h2,4-6,9H,3H2,1H3,(H2,13,15)(H,14,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144336

(3-(6-Amino-5-methyl-pyridin-3-yl)-2-[1-(3-methyl-b...)Show InChI InChI=1S/C17H24N4O2/c1-11(2)4-5-21-9-15(20-10-21)14(17(22)23)7-13-6-12(3)16(18)19-8-13/h6,8-11,14H,4-5,7H2,1-3H3,(H2,18,19)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144317
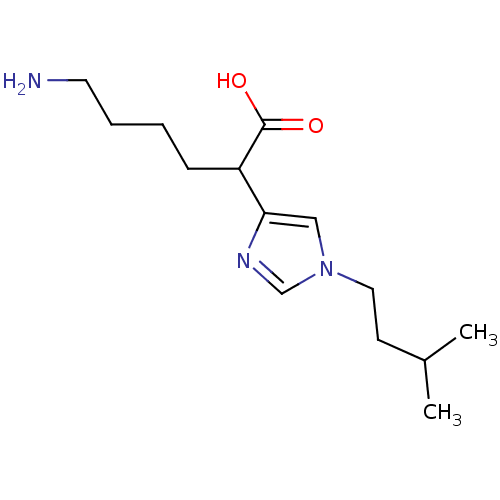
(6-Amino-2-[1-(3-methyl-butyl)-1H-imidazol-4-yl]-he...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)6-8-17-9-13(16-10-17)12(14(18)19)5-3-4-7-15/h9-12H,3-8,15H2,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50135933
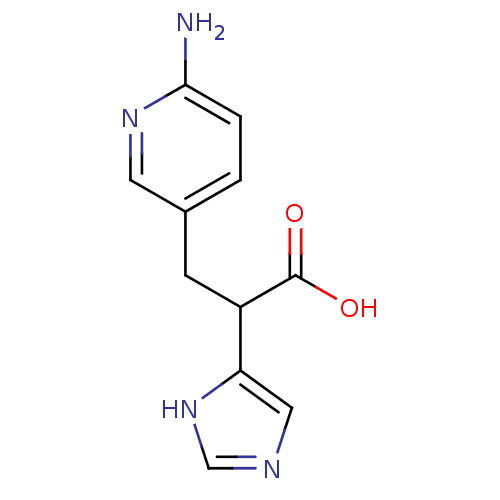
(3-(6-Amino-pyridin-3-yl)-2-(1H-imidazol-4-yl)-prop...)Show InChI InChI=1S/C11H12N4O2/c12-10-2-1-7(4-14-10)3-8(11(16)17)9-5-13-6-15-9/h1-2,4-6,8H,3H2,(H2,12,14)(H,13,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135933

(3-(6-Amino-pyridin-3-yl)-2-(1H-imidazol-4-yl)-prop...)Show InChI InChI=1S/C11H12N4O2/c12-10-2-1-7(4-14-10)3-8(11(16)17)9-5-13-6-15-9/h1-2,4-6,8H,3H2,(H2,12,14)(H,13,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144316

(3-(6-Amino-5-methyl-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C12H14N4O2/c1-7-2-8(4-15-11(7)13)3-9(12(17)18)10-5-14-6-16-10/h2,4-6,9H,3H2,1H3,(H2,13,15)(H,14,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144325
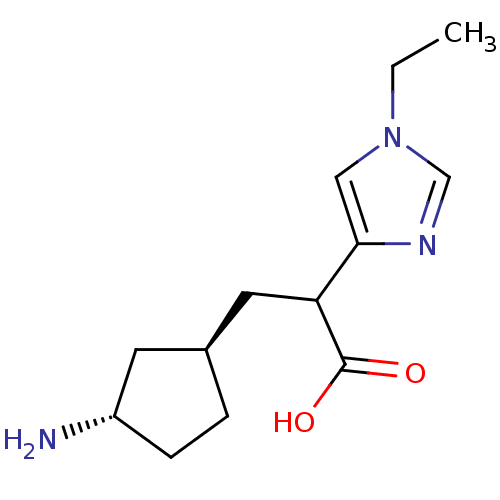
(3-((1R,3S)-3-Amino-cyclopentyl)-2-(1-ethyl-1H-imid...)Show InChI InChI=1S/C13H21N3O2/c1-2-16-7-12(15-8-16)11(13(17)18)6-9-3-4-10(14)5-9/h7-11H,2-6,14H2,1H3,(H,17,18)/t9-,10-,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144325

(3-((1R,3S)-3-Amino-cyclopentyl)-2-(1-ethyl-1H-imid...)Show InChI InChI=1S/C13H21N3O2/c1-2-16-7-12(15-8-16)11(13(17)18)6-9-3-4-10(14)5-9/h7-11H,2-6,14H2,1H3,(H,17,18)/t9-,10-,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144317

(6-Amino-2-[1-(3-methyl-butyl)-1H-imidazol-4-yl]-he...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)6-8-17-9-13(16-10-17)12(14(18)19)5-3-4-7-15/h9-12H,3-8,15H2,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144322
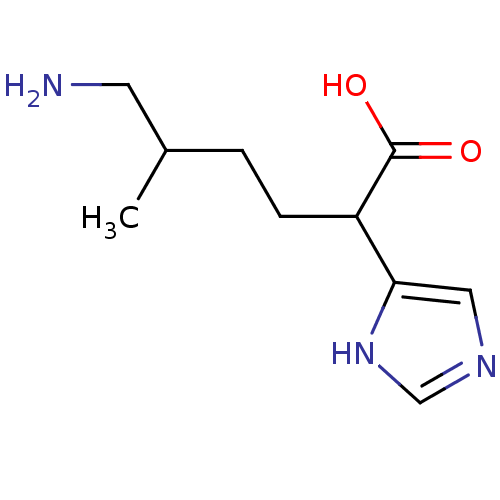
(6-Amino-2-(1H-imidazol-4-yl)-5-methyl-hexanoic aci...)Show InChI InChI=1S/C10H17N3O2/c1-7(4-11)2-3-8(10(14)15)9-5-12-6-13-9/h5-8H,2-4,11H2,1H3,(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144320
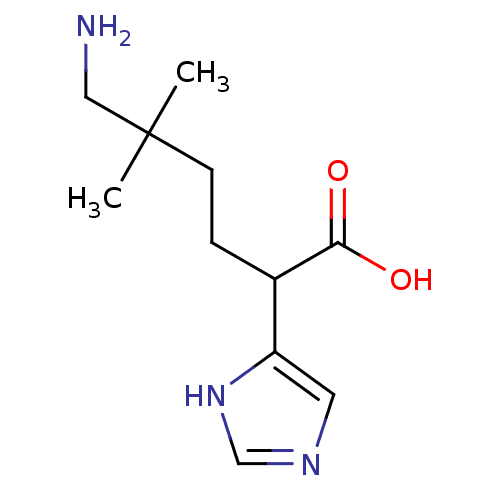
(6-Amino-2-(1H-imidazol-4-yl)-5,5-dimethyl-hexanoic...)Show InChI InChI=1S/C11H19N3O2/c1-11(2,6-12)4-3-8(10(15)16)9-5-13-7-14-9/h5,7-8H,3-4,6,12H2,1-2H3,(H,13,14)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144334
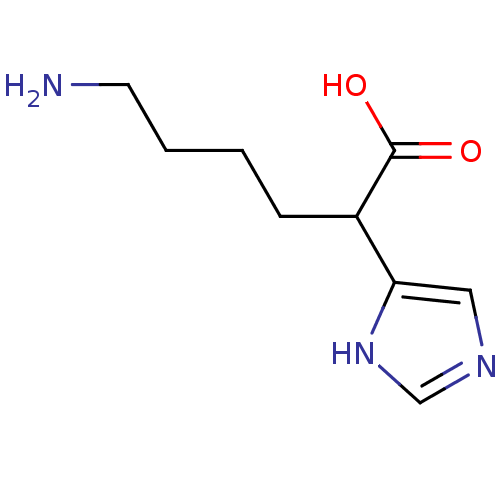
(6-Amino-2-(1H-imidazol-4-yl)-hexanoic acid | CHEMB...)Show InChI InChI=1S/C9H15N3O2/c10-4-2-1-3-7(9(13)14)8-5-11-6-12-8/h5-7H,1-4,10H2,(H,11,12)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144320

(6-Amino-2-(1H-imidazol-4-yl)-5,5-dimethyl-hexanoic...)Show InChI InChI=1S/C11H19N3O2/c1-11(2,6-12)4-3-8(10(15)16)9-5-13-7-14-9/h5,7-8H,3-4,6,12H2,1-2H3,(H,13,14)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144322

(6-Amino-2-(1H-imidazol-4-yl)-5-methyl-hexanoic aci...)Show InChI InChI=1S/C10H17N3O2/c1-7(4-11)2-3-8(10(14)15)9-5-12-6-13-9/h5-8H,2-4,11H2,1H3,(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144323
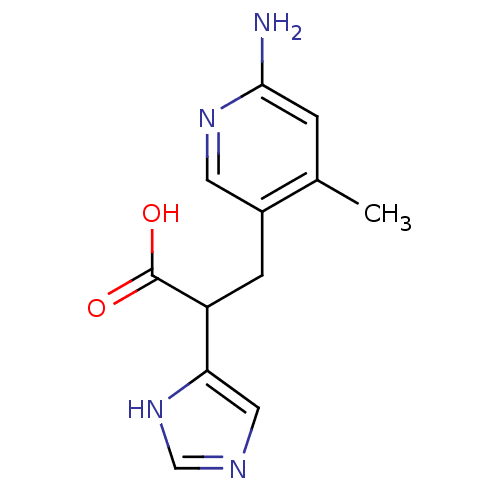
(3-(6-Amino-4-methyl-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C12H14N4O2/c1-7-2-11(13)15-4-8(7)3-9(12(17)18)10-5-14-6-16-10/h2,4-6,9H,3H2,1H3,(H2,13,15)(H,14,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144329
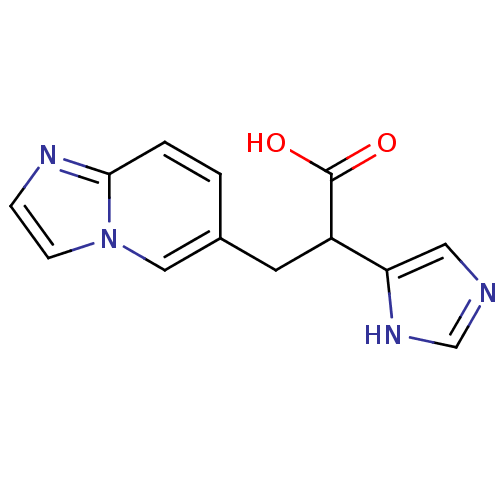
(3-Imidazo[1,2-a]pyridin-6-yl-2-(1H-imidazol-4-yl)-...)Show InChI InChI=1S/C13H12N4O2/c18-13(19)10(11-6-14-8-16-11)5-9-1-2-12-15-3-4-17(12)7-9/h1-4,6-8,10H,5H2,(H,14,16)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144334

(6-Amino-2-(1H-imidazol-4-yl)-hexanoic acid | CHEMB...)Show InChI InChI=1S/C9H15N3O2/c10-4-2-1-3-7(9(13)14)8-5-11-6-12-8/h5-7H,1-4,10H2,(H,11,12)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144324
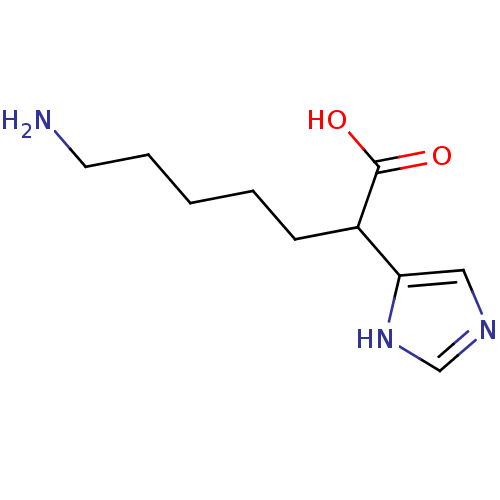
(7-Amino-2-(1H-imidazol-4-yl)-heptanoic acid | CHEM...)Show InChI InChI=1S/C10H17N3O2/c11-5-3-1-2-4-8(10(14)15)9-6-12-7-13-9/h6-8H,1-5,11H2,(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144332
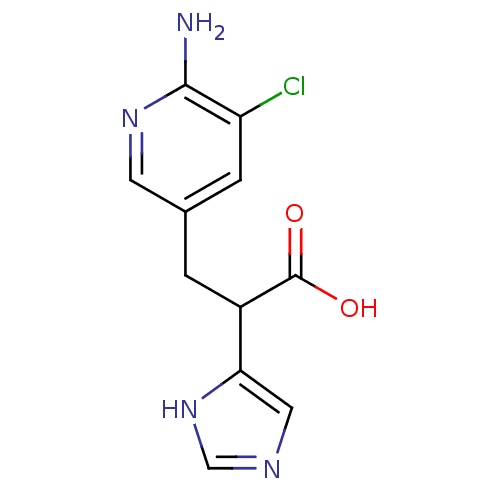
(3-(6-Amino-5-chloro-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C11H11ClN4O2/c12-8-2-6(3-15-10(8)13)1-7(11(17)18)9-4-14-5-16-9/h2-5,7H,1H2,(H2,13,15)(H,14,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144339
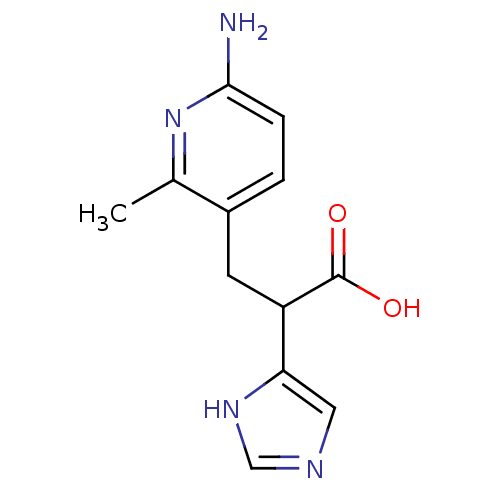
(3-(6-Amino-2-methyl-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C12H14N4O2/c1-7-8(2-3-11(13)16-7)4-9(12(17)18)10-5-14-6-15-10/h2-3,5-6,9H,4H2,1H3,(H2,13,16)(H,14,15)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144327
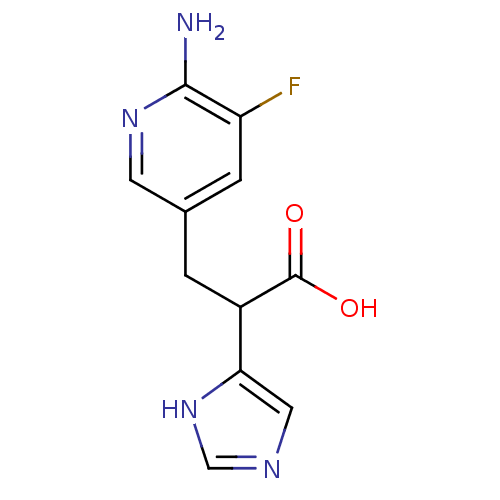
(3-(6-Amino-5-fluoro-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C11H11FN4O2/c12-8-2-6(3-15-10(8)13)1-7(11(17)18)9-4-14-5-16-9/h2-5,7H,1H2,(H2,13,15)(H,14,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144321
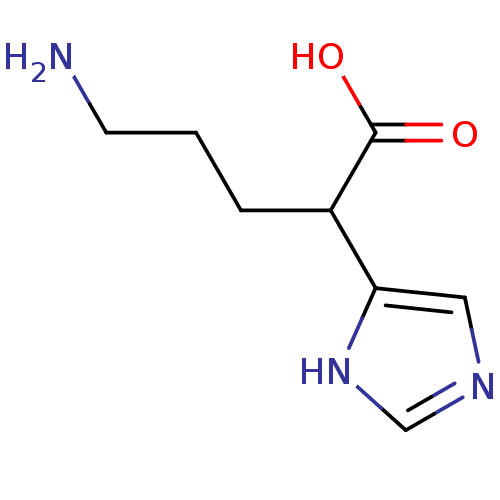
(5-Amino-2-(1H-imidazol-4-yl)-pentanoic acid | CHEM...)Show InChI InChI=1S/C8H13N3O2/c9-3-1-2-6(8(12)13)7-4-10-5-11-7/h4-6H,1-3,9H2,(H,10,11)(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144341
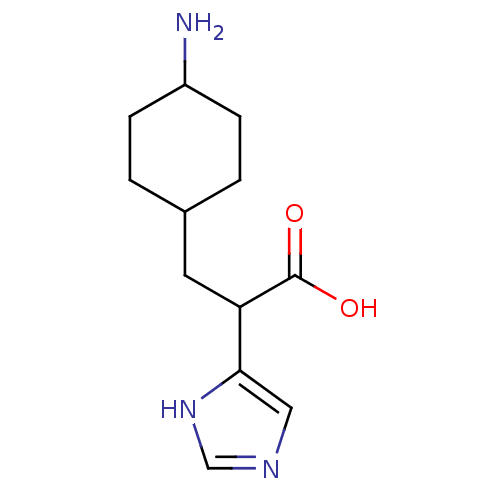
(3-(4-Amino-cyclohexyl)-2-(1H-imidazol-4-yl)-propio...)Show SMILES NC1CCC(CC(C(O)=O)c2cnc[nH]2)CC1 |(5.49,-.09,;6.82,-.85,;8.17,-.09,;9.5,-.85,;9.5,-2.41,;10.84,-3.18,;12.36,-2.93,;12.92,-1.49,;14.43,-1.24,;11.94,-.29,;13.33,-4.13,;12.94,-5.62,;14.24,-6.46,;15.43,-5.49,;14.88,-4.05,;8.17,-3.18,;6.84,-2.41,)| Show InChI InChI=1S/C12H19N3O2/c13-9-3-1-8(2-4-9)5-10(12(16)17)11-6-14-7-15-11/h6-10H,1-5,13H2,(H,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144330

(3-(2-Amino-pyridin-4-yl)-2-(1H-imidazol-4-yl)-prop...)Show InChI InChI=1S/C11H12N4O2/c12-10-4-7(1-2-14-10)3-8(11(16)17)9-5-13-6-15-9/h1-2,4-6,8H,3H2,(H2,12,14)(H,13,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144318
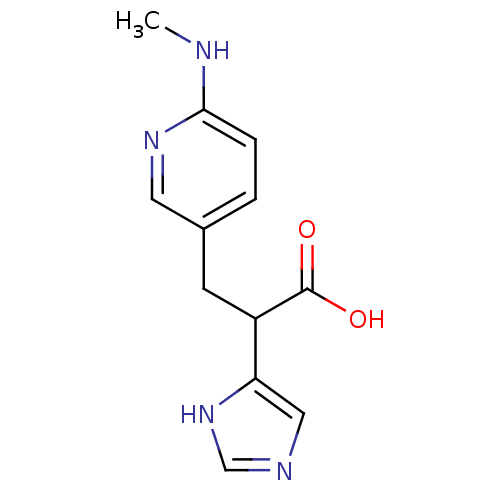
(2-(1H-Imidazol-4-yl)-3-(6-methylamino-pyridin-3-yl...)Show InChI InChI=1S/C12H14N4O2/c1-13-11-3-2-8(5-15-11)4-9(12(17)18)10-6-14-7-16-10/h2-3,5-7,9H,4H2,1H3,(H,13,15)(H,14,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144343

(3-(6-Amino-pyridin-2-yl)-2-(1H-imidazol-4-yl)-prop...)Show InChI InChI=1S/C11H12N4O2/c12-10-3-1-2-7(15-10)4-8(11(16)17)9-5-13-6-14-9/h1-3,5-6,8H,4H2,(H2,12,15)(H,13,14)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144331
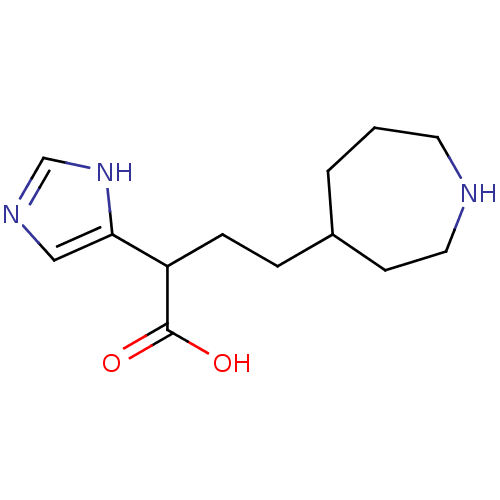
(4-Azepan-4-yl-2-(1H-imidazol-4-yl)-butyric acid | ...)Show InChI InChI=1S/C13H21N3O2/c17-13(18)11(12-8-15-9-16-12)4-3-10-2-1-6-14-7-5-10/h8-11,14H,1-7H2,(H,15,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144345
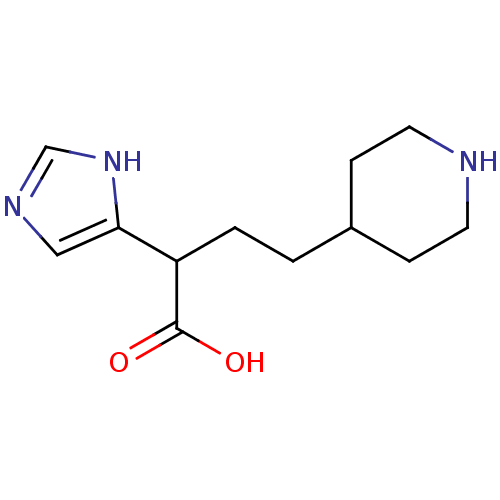
(2-(1H-Imidazol-4-yl)-4-piperidin-4-yl-butyric acid...)Show InChI InChI=1S/C12H19N3O2/c16-12(17)10(11-7-14-8-15-11)2-1-9-3-5-13-6-4-9/h7-10,13H,1-6H2,(H,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144344
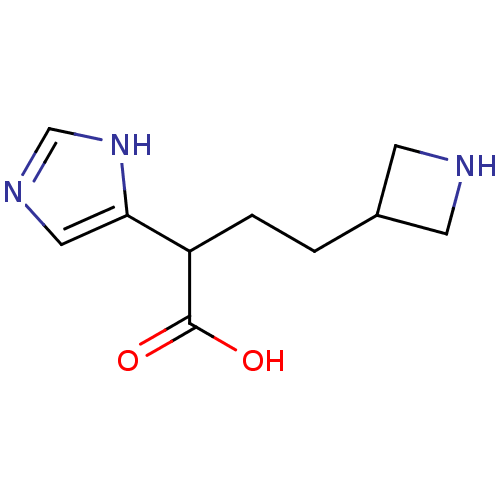
(4-Azetidin-3-yl-2-(1H-imidazol-4-yl)-butyric acid ...)Show InChI InChI=1S/C10H15N3O2/c14-10(15)8(9-5-12-6-13-9)2-1-7-3-11-4-7/h5-8,11H,1-4H2,(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144315
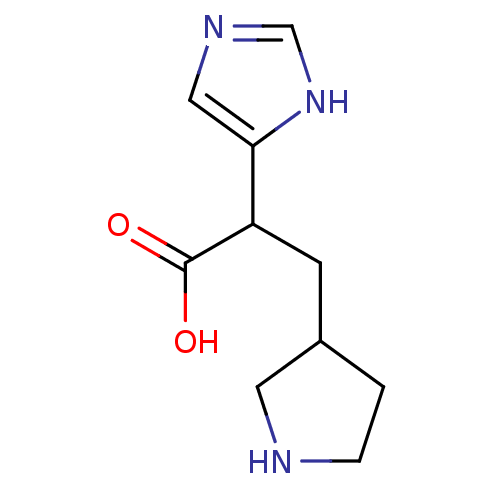
(2-(1H-Imidazol-4-yl)-3-pyrrolidin-3-yl-propionic a...)Show InChI InChI=1S/C10H15N3O2/c14-10(15)8(9-5-12-6-13-9)3-7-1-2-11-4-7/h5-8,11H,1-4H2,(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144340
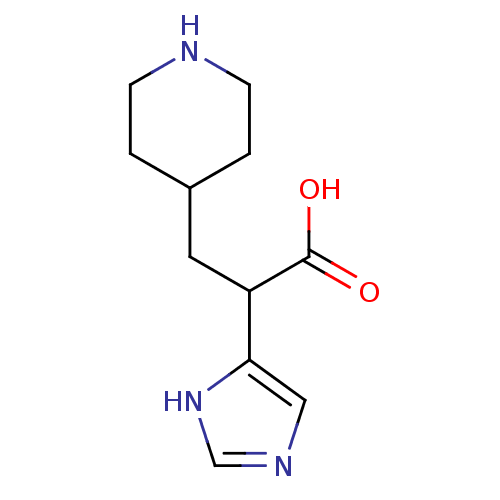
(2-(1H-Imidazol-4-yl)-3-piperidin-4-yl-propionic ac...)Show InChI InChI=1S/C11H17N3O2/c15-11(16)9(10-6-13-7-14-10)5-8-1-3-12-4-2-8/h6-9,12H,1-5H2,(H,13,14)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144346
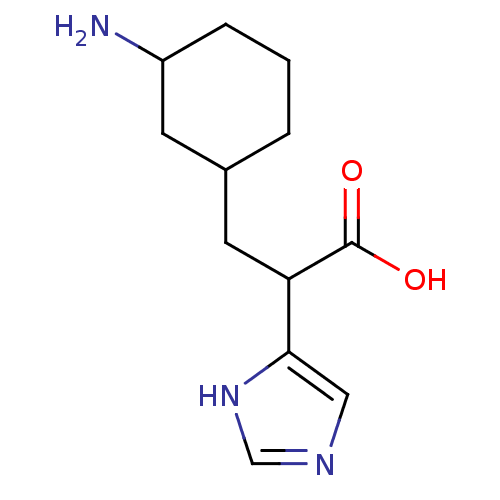
(3-(3-Amino-cyclohexyl)-2-(1H-imidazol-4-yl)-propio...)Show InChI InChI=1S/C12H19N3O2/c13-9-3-1-2-8(4-9)5-10(12(16)17)11-6-14-7-15-11/h6-10H,1-5,13H2,(H,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144328
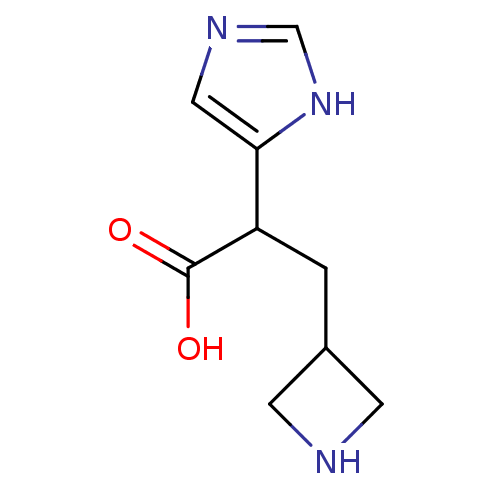
(3-Azetidin-3-yl-2-(1H-imidazol-4-yl)-propionic aci...)Show InChI InChI=1S/C9H13N3O2/c13-9(14)7(1-6-2-10-3-6)8-4-11-5-12-8/h4-7,10H,1-3H2,(H,11,12)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144335

(2-(1H-Imidazol-4-yl)-4-pyrrolidin-3-yl-butyric aci...)Show InChI InChI=1S/C11H17N3O2/c15-11(16)9(10-6-13-7-14-10)2-1-8-3-4-12-5-8/h6-9,12H,1-5H2,(H,13,14)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144347
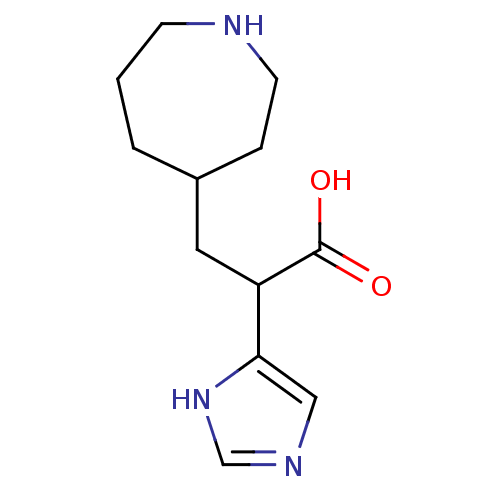
(3-Azepan-4-yl-2-(1H-imidazol-4-yl)-propionic acid ...)Show InChI InChI=1S/C12H19N3O2/c16-12(17)10(11-7-14-8-15-11)6-9-2-1-4-13-5-3-9/h7-10,13H,1-6H2,(H,14,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144319

(2-(1H-Imidazol-4-yl)-6-methylamino-hexanoic acid |...)Show InChI InChI=1S/C10H17N3O2/c1-11-5-3-2-4-8(10(14)15)9-6-12-7-13-9/h6-8,11H,2-5H2,1H3,(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144338
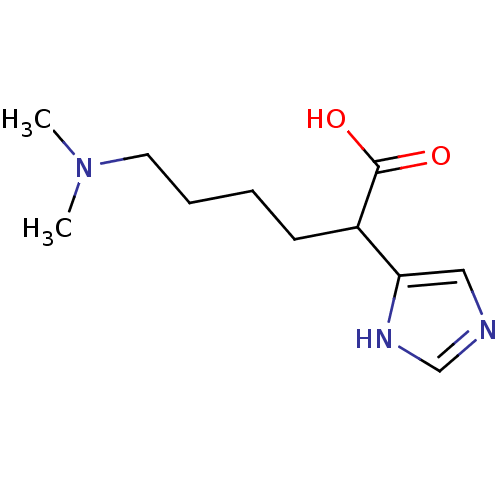
(6-Dimethylamino-2-(1H-imidazol-4-yl)-hexanoic acid...)Show InChI InChI=1S/C11H19N3O2/c1-14(2)6-4-3-5-9(11(15)16)10-7-12-8-13-10/h7-9H,3-6H2,1-2H3,(H,12,13)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144326
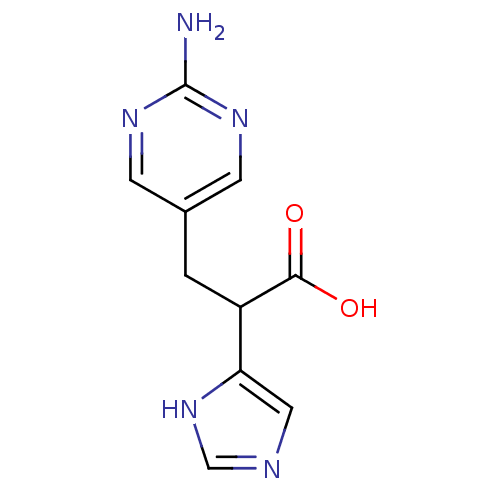
(3-(2-Amino-pyrimidin-5-yl)-2-(1H-imidazol-4-yl)-pr...)Show InChI InChI=1S/C10H11N5O2/c11-10-13-2-6(3-14-10)1-7(9(16)17)8-4-12-5-15-8/h2-5,7H,1H2,(H,12,15)(H,16,17)(H2,11,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data