

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Rattus norvegicus) | BDBM50144378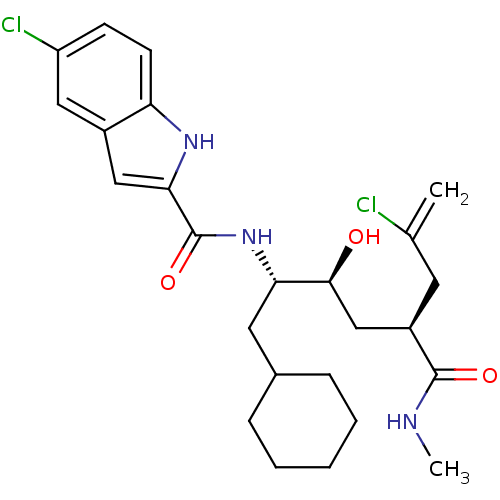 (5-Chloro-1H-indole-2-carboxylic acid ((1S,2S,4S)-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of compound against renin was determined | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50144376 (CHEMBL69411 | Quinoline-3-carboxylic acid ((1S,2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CCL3 binding to C-C chemokine receptor type 1 | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50144371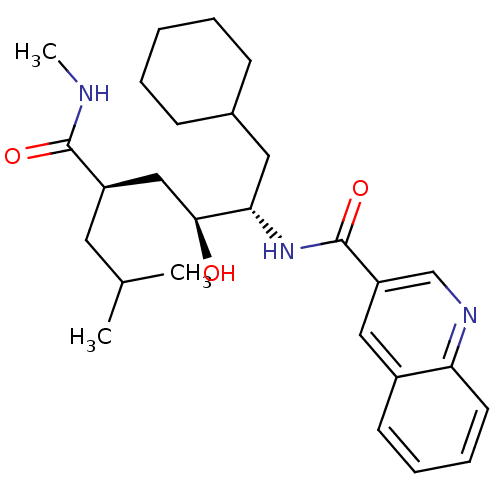 (CHEMBL68937 | Quinoline-3-carboxylic acid ((1S,2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CCL3 binding to C-C chemokine receptor type 1 | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50144373 (CHEMBL67809 | Quinoline-3-carboxylic acid ((1S,2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CCL3 binding to C-C chemokine receptor type 1 | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Rattus norvegicus) | BDBM50144371 (CHEMBL68937 | Quinoline-3-carboxylic acid ((1S,2S,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory activity of compound against renin was determined | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50144383 (CHEMBL308690 | Quinoline-3-carboxylic acid ((1S,2S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CCL3 binding to C-C chemokine receptor type 1 | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50144379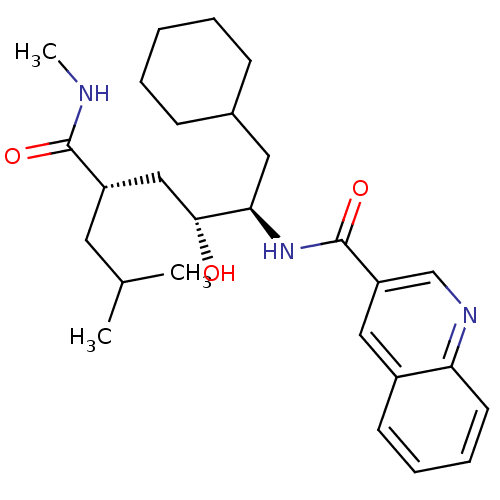 (CHEMBL69822 | Quinoline-3-carboxylic acid ((1R,2R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CCL3 binding to C-C chemokine receptor type 1 | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50144384 (CHEMBL308243 | Quinoline-3-carboxylic acid ((1S,2S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CCL3 binding to C-C chemokine receptor type 1 | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50144378 (5-Chloro-1H-indole-2-carboxylic acid ((1S,2S,4S)-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of CCL3 binding to C-C chemokine receptor type 1 | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50219834 (CHEMBL303623) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for inhibition of CCL3 binding to chemokine receptor-1; inactive at a concentration of 32 uM | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50219341 (CHEMBL70208) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for inhibition of CCL3 binding to chemokine receptor-1; inactive at a concentration of 32 uM | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50219441 (CHEMBL69900) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for inhibition of CCL3 binding to chemokine receptor-1; inactive at a concentration of 32 uM | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50219440 (CHEMBL68602) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for inhibition of CCL3 binding to chemokine receptor-1; inactive at a concentration of 32 uM | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50219952 (CHEMBL430598) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for inhibition of CCL3 binding to chemokine receptor-1; inactive at a concentration of 32 uM | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50219951 (CHEMBL71965) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for inhibition of CCL3 binding to chemokine receptor-1; inactive at a concentration of 32 uM | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50219833 (CHEMBL305065) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for inhibition of CCL3 binding to chemokine receptor-1; inactive at a concentration of 32 uM | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50219832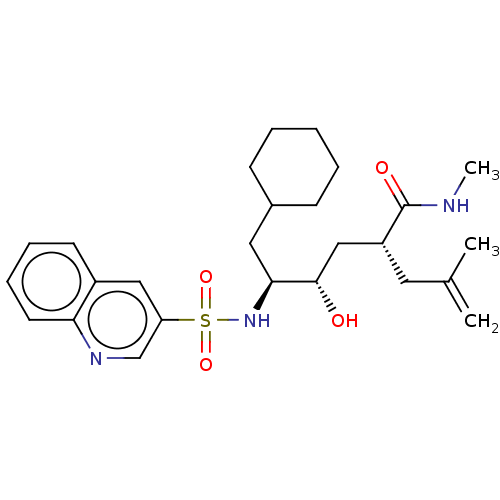 (CHEMBL69619) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was tested for inhibition of CCL3 binding to chemokine receptor-1; inactive at a concentration of 32 uM | Bioorg Med Chem Lett 14: 2163-7 (2004) Article DOI: 10.1016/j.bmcl.2004.02.020 BindingDB Entry DOI: 10.7270/Q2CV4H5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||