 Found 35 hits of Enzyme Inhibition Constant Data
Found 35 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144535
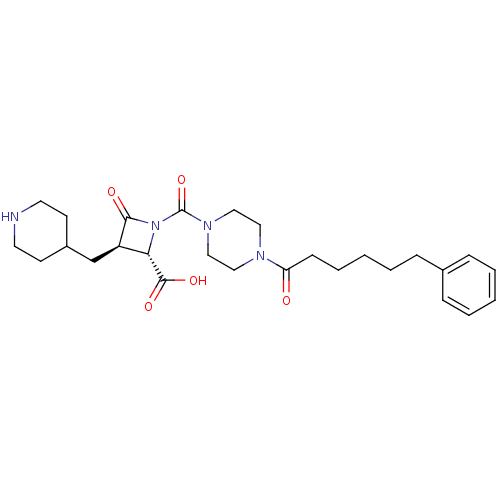
((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C27H38N4O5/c32-23(10-6-2-5-9-20-7-3-1-4-8-20)29-15-17-30(18-16-29)27(36)31-24(26(34)35)22(25(31)33)19-21-11-13-28-14-12-21/h1,3-4,7-8,21-22,24,28H,2,5-6,9-19H2,(H,34,35)/t22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217824
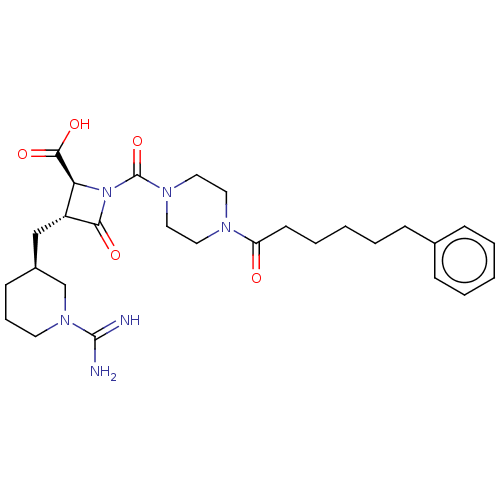
(BMS-363130 | CHEMBL70738)Show SMILES [H][C@@]1(C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)CCCCCc3ccccc3)C2=O)C(O)=O)CCCN(C1)C(N)=N Show InChI InChI=1S/C28H40N6O5/c29-27(30)33-13-7-11-21(19-33)18-22-24(26(37)38)34(25(22)36)28(39)32-16-14-31(15-17-32)23(35)12-6-2-5-10-20-8-3-1-4-9-20/h1,3-4,8-9,21-22,24H,2,5-7,10-19H2,(H3,29,30)(H,37,38)/t21-,22+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50221046
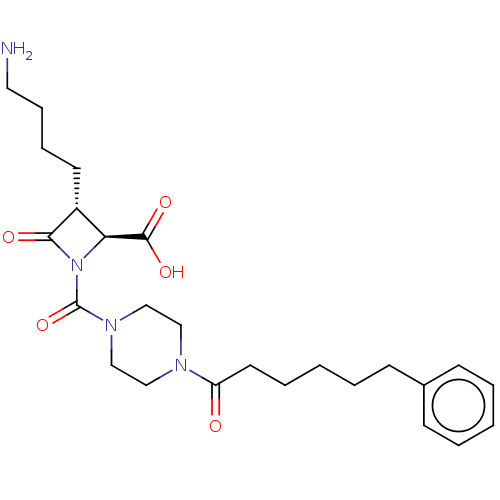
(CHEMBL72282)Show SMILES NCCCC[C@@H]1[C@H](N(C(=O)N2CCN(CC2)C(=O)CCCCCc2ccccc2)C1=O)C(O)=O Show InChI InChI=1S/C25H36N4O5/c26-14-8-7-12-20-22(24(32)33)29(23(20)31)25(34)28-17-15-27(16-18-28)21(30)13-6-2-5-11-19-9-3-1-4-10-19/h1,3-4,9-10,20,22H,2,5-8,11-18,26H2,(H,32,33)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50120387
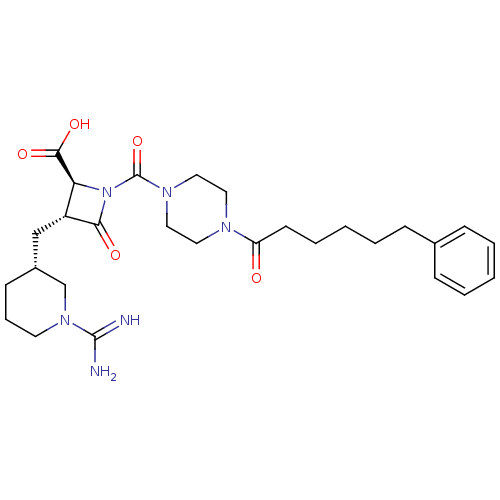
((2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethy...)Show SMILES NC(=N)N1CCC[C@H](C[C@@H]2[C@H](N(C(=O)N3CCN(CC3)C(=O)CCCCCc3ccccc3)C2=O)C(O)=O)C1 Show InChI InChI=1S/C28H40N6O5/c29-27(30)33-13-7-11-21(19-33)18-22-24(26(37)38)34(25(22)36)28(39)32-16-14-31(15-17-32)23(35)12-6-2-5-10-20-8-3-1-4-9-20/h1,3-4,8-9,21-22,24H,2,5-7,10-19H2,(H3,29,30)(H,37,38)/t21-,22-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144532
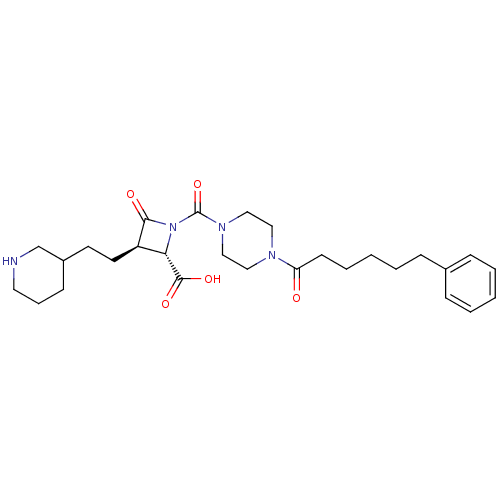
((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CCC2CCCNC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C28H40N4O5/c33-24(12-6-2-5-10-21-8-3-1-4-9-21)30-16-18-31(19-17-30)28(37)32-25(27(35)36)23(26(32)34)14-13-22-11-7-15-29-20-22/h1,3-4,8-9,22-23,25,29H,2,5-7,10-20H2,(H,35,36)/t22?,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220841
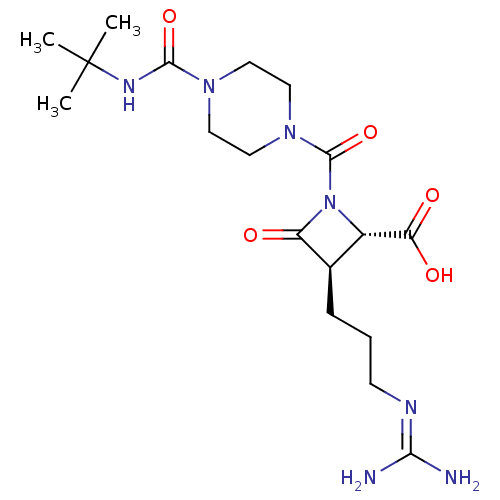
(BMS-262084 | CHEMBL71037)Show SMILES [#6]C([#6])([#6])[#7]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6@@H](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]-1=O)-[#6](-[#8])=O Show InChI InChI=1S/C18H31N7O5/c1-18(2,3)22-16(29)23-7-9-24(10-8-23)17(30)25-12(14(27)28)11(13(25)26)5-4-6-21-15(19)20/h11-12H,4-10H2,1-3H3,(H,22,29)(H,27,28)(H4,19,20,21)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50217813
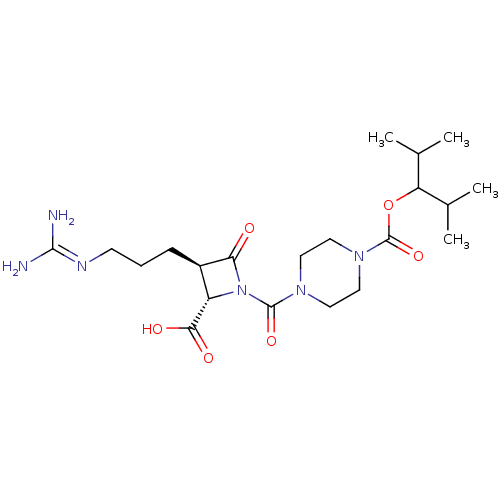
(CHEMBL302058)Show SMILES [#6]-[#6](-[#6])-[#6](-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6@@H](-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]-1=O)-[#6](-[#8])=O)-[#6](-[#6])-[#6] Show InChI InChI=1S/C21H36N6O6/c1-12(2)16(13(3)4)33-21(32)26-10-8-25(9-11-26)20(31)27-15(18(29)30)14(17(27)28)6-5-7-24-19(22)23/h12-16H,5-11H2,1-4H3,(H,29,30)(H4,22,23,24)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220827

(CHEMBL441447)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CCCCN)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C21H36N4O6/c1-13(2)17(14(3)4)31-21(30)24-11-9-23(10-12-24)20(29)25-16(19(27)28)15(18(25)26)7-5-6-8-22/h13-17H,5-12,22H2,1-4H3,(H,27,28)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220830

(CHEMBL306696)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](Cc2cccc(CN)c2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C25H36N4O6/c1-15(2)21(16(3)4)35-25(34)28-10-8-27(9-11-28)24(33)29-20(23(31)32)19(22(29)30)13-17-6-5-7-18(12-17)14-26/h5-7,12,15-16,19-21H,8-11,13-14,26H2,1-4H3,(H,31,32)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50144531
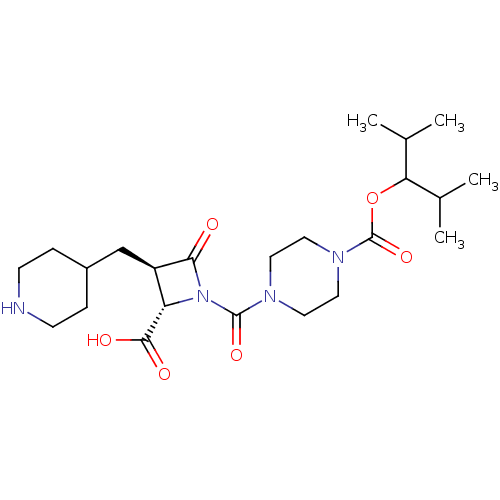
(4-((2S,3R)-2-Carboxy-4-oxo-3-piperidin-4-ylmethyl-...)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCNCC2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H38N4O6/c1-14(2)19(15(3)4)33-23(32)26-11-9-25(10-12-26)22(31)27-18(21(29)30)17(20(27)28)13-16-5-7-24-8-6-16/h14-19,24H,5-13H2,1-4H3,(H,29,30)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220737
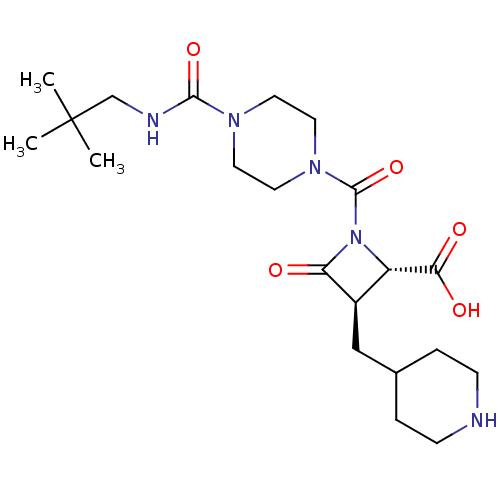
(CHEMBL308109)Show SMILES CC(C)(C)CNC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCNCC2)C1=O)C(O)=O Show InChI InChI=1S/C21H35N5O5/c1-21(2,3)13-23-19(30)24-8-10-25(11-9-24)20(31)26-16(18(28)29)15(17(26)27)12-14-4-6-22-7-5-14/h14-16,22H,4-13H2,1-3H3,(H,23,30)(H,28,29)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50221047

(CHEMBL70529)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CCCCCCN)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H40N4O6/c1-15(2)19(16(3)4)33-23(32)26-13-11-25(12-14-26)22(31)27-18(21(29)30)17(20(27)28)9-7-5-6-8-10-24/h15-19H,5-14,24H2,1-4H3,(H,29,30)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220837
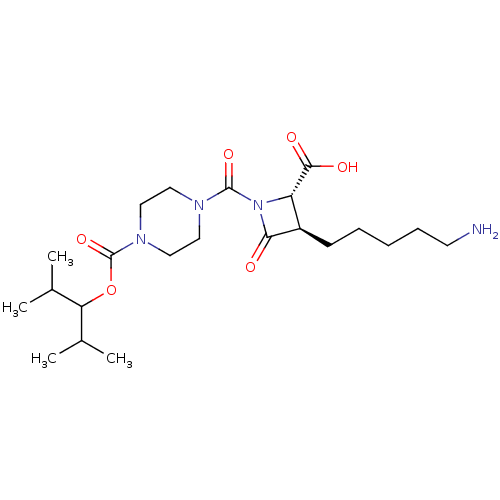
(CHEMBL70666)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CCCCCN)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C22H38N4O6/c1-14(2)18(15(3)4)32-22(31)25-12-10-24(11-13-25)21(30)26-17(20(28)29)16(19(26)27)8-6-5-7-9-23/h14-18H,5-13,23H2,1-4H3,(H,28,29)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220831
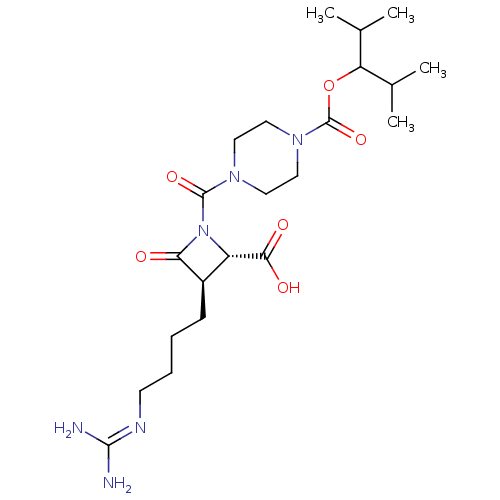
(CHEMBL70964)Show SMILES [#6]-[#6](-[#6])-[#6](-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#7]-1-[#6@@H](-[#6@@H](-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6]-1=O)-[#6](-[#8])=O)-[#6](-[#6])-[#6] Show InChI InChI=1S/C22H38N6O6/c1-13(2)17(14(3)4)34-22(33)27-11-9-26(10-12-27)21(32)28-16(19(30)31)15(18(28)29)7-5-6-8-25-20(23)24/h13-17H,5-12H2,1-4H3,(H,30,31)(H4,23,24,25)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50221048
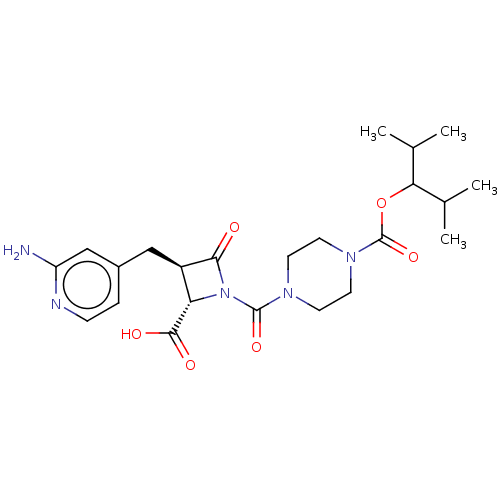
(CHEMBL422412)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](Cc2ccnc(N)c2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H33N5O6/c1-13(2)19(14(3)4)34-23(33)27-9-7-26(8-10-27)22(32)28-18(21(30)31)16(20(28)29)11-15-5-6-25-17(24)12-15/h5-6,12-14,16,18-19H,7-11H2,1-4H3,(H2,24,25)(H,30,31)/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220825
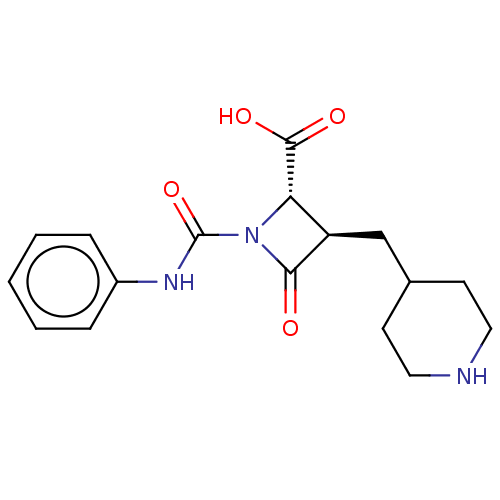
(CHEMBL305950)Show SMILES OC(=O)[C@@H]1[C@@H](CC2CCNCC2)C(=O)N1C(=O)Nc1ccccc1 Show InChI InChI=1S/C17H21N3O4/c21-15-13(10-11-6-8-18-9-7-11)14(16(22)23)20(15)17(24)19-12-4-2-1-3-5-12/h1-5,11,13-14,18H,6-10H2,(H,19,24)(H,22,23)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220739

(CHEMBL430999)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](Cc2ccc(CN)cc2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C25H36N4O6/c1-15(2)21(16(3)4)35-25(34)28-11-9-27(10-12-28)24(33)29-20(23(31)32)19(22(29)30)13-17-5-7-18(14-26)8-6-17/h5-8,15-16,19-21H,9-14,26H2,1-4H3,(H,31,32)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Rattus norvegicus) | BDBM50144532

((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CCC2CCCNC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C28H40N4O5/c33-24(12-6-2-5-10-21-8-3-1-4-9-21)30-16-18-31(19-17-30)28(37)32-25(27(35)36)23(26(32)34)14-13-22-11-7-15-29-20-22/h1,3-4,8-9,22-23,25,29H,2,5-7,10-20H2,(H,35,36)/t22?,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 309 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Plasmin was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220829
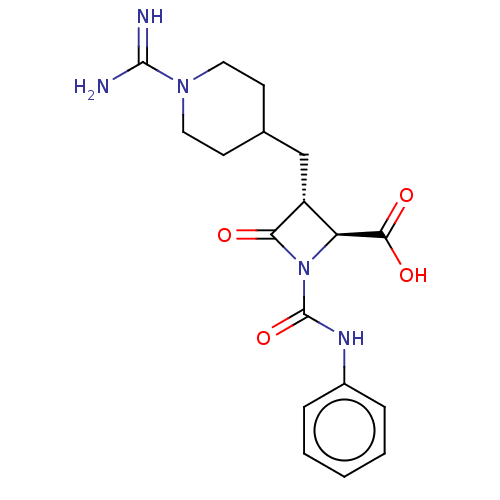
(CHEMBL307089)Show SMILES NC(=N)N1CCC(C[C@@H]2[C@H](N(C(=O)Nc3ccccc3)C2=O)C(O)=O)CC1 Show InChI InChI=1S/C18H23N5O4/c19-17(20)22-8-6-11(7-9-22)10-13-14(16(25)26)23(15(13)24)18(27)21-12-4-2-1-3-5-12/h1-5,11,13-14H,6-10H2,(H3,19,20)(H,21,27)(H,25,26)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 515 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50144531

(4-((2S,3R)-2-Carboxy-4-oxo-3-piperidin-4-ylmethyl-...)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCNCC2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H38N4O6/c1-14(2)19(15(3)4)33-23(32)26-11-9-25(10-12-26)22(31)27-18(21(29)30)17(20(27)28)13-16-5-7-24-8-6-16/h14-19,24H,5-13H2,1-4H3,(H,29,30)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 935 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Trypsin was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220828

(CHEMBL70619)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](Cc2ccc(N)nc2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H33N5O6/c1-13(2)19(14(3)4)34-23(33)27-9-7-26(8-10-27)22(32)28-18(21(30)31)16(20(28)29)11-15-5-6-17(24)25-12-15/h5-6,12-14,16,18-19H,7-11H2,1-4H3,(H2,24,25)(H,30,31)/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50221013
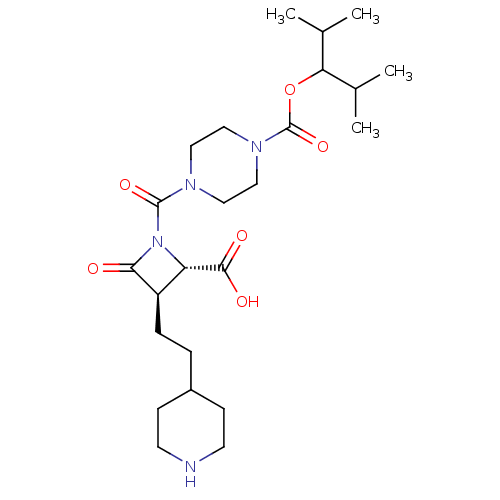
(CHEMBL273331)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CCC2CCNCC2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C24H40N4O6/c1-15(2)20(16(3)4)34-24(33)27-13-11-26(12-14-27)23(32)28-19(22(30)31)18(21(28)29)6-5-17-7-9-25-10-8-17/h15-20,25H,5-14H2,1-4H3,(H,30,31)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50221012
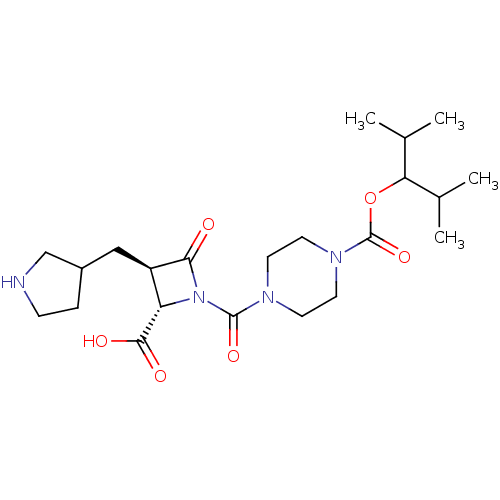
(CHEMBL308015)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCNC2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C22H36N4O6/c1-13(2)18(14(3)4)32-22(31)25-9-7-24(8-10-25)21(30)26-17(20(28)29)16(19(26)27)11-15-5-6-23-12-15/h13-18,23H,5-12H2,1-4H3,(H,28,29)/t15?,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Rattus norvegicus) | BDBM50144531

(4-((2S,3R)-2-Carboxy-4-oxo-3-piperidin-4-ylmethyl-...)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCNCC2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H38N4O6/c1-14(2)19(15(3)4)33-23(32)26-11-9-25(10-12-26)22(31)27-18(21(29)30)17(20(27)28)13-16-5-7-24-8-6-16/h14-19,24H,5-13H2,1-4H3,(H,29,30)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Plasmin was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50144531

(4-((2S,3R)-2-Carboxy-4-oxo-3-piperidin-4-ylmethyl-...)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCNCC2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H38N4O6/c1-14(2)19(15(3)4)33-23(32)26-11-9-25(10-12-26)22(31)27-18(21(29)30)17(20(27)28)13-16-5-7-24-8-6-16/h14-19,24H,5-13H2,1-4H3,(H,29,30)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Urokinase-type plasminogen activator was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50144532

((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CCC2CCCNC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C28H40N4O5/c33-24(12-6-2-5-10-21-8-3-1-4-9-21)30-16-18-31(19-17-30)28(37)32-25(27(35)36)23(26(32)34)14-13-22-11-7-15-29-20-22/h1,3-4,8-9,22-23,25,29H,2,5-7,10-20H2,(H,35,36)/t22?,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Trypsin was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50221014
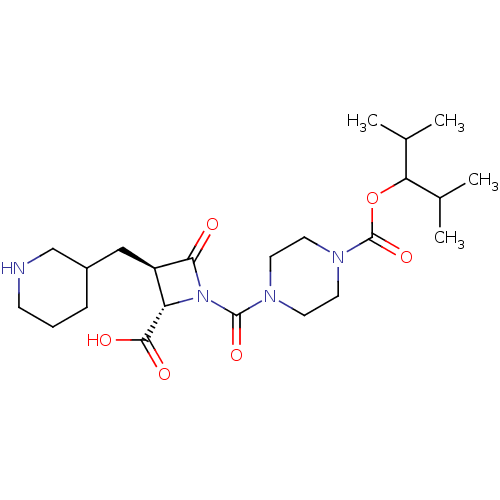
(CHEMBL70665)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCCNC2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H38N4O6/c1-14(2)19(15(3)4)33-23(32)26-10-8-25(9-11-26)22(31)27-18(21(29)30)17(20(27)28)12-16-6-5-7-24-13-16/h14-19,24H,5-13H2,1-4H3,(H,29,30)/t16?,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50144531

(4-((2S,3R)-2-Carboxy-4-oxo-3-piperidin-4-ylmethyl-...)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCNCC2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H38N4O6/c1-14(2)19(15(3)4)33-23(32)26-11-9-25(10-12-26)22(31)27-18(21(29)30)17(20(27)28)13-16-5-7-24-8-6-16/h14-19,24H,5-13H2,1-4H3,(H,29,30)/t17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Thrombin was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50144532

((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CCC2CCCNC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C28H40N4O5/c33-24(12-6-2-5-10-21-8-3-1-4-9-21)30-16-18-31(19-17-30)28(37)32-25(27(35)36)23(26(32)34)14-13-22-11-7-15-29-20-22/h1,3-4,8-9,22-23,25,29H,2,5-7,10-20H2,(H,35,36)/t22?,23-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Thrombin was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50144531

(4-((2S,3R)-2-Carboxy-4-oxo-3-piperidin-4-ylmethyl-...)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCNCC2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H38N4O6/c1-14(2)19(15(3)4)33-23(32)26-11-9-25(10-12-26)22(31)27-18(21(29)30)17(20(27)28)13-16-5-7-24-8-6-16/h14-19,24H,5-13H2,1-4H3,(H,29,30)/t17-,18+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against tissue type plasminogen activator was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50144532

((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CCC2CCCNC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C28H40N4O5/c33-24(12-6-2-5-10-21-8-3-1-4-9-21)30-16-18-31(19-17-30)28(37)32-25(27(35)36)23(26(32)34)14-13-22-11-7-15-29-20-22/h1,3-4,8-9,22-23,25,29H,2,5-7,10-20H2,(H,35,36)/t22?,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Urokinase-type plasminogen activator was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144532

((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CCC2CCCNC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C28H40N4O5/c33-24(12-6-2-5-10-21-8-3-1-4-9-21)30-16-18-31(19-17-30)28(37)32-25(27(35)36)23(26(32)34)14-13-22-11-7-15-29-20-22/h1,3-4,8-9,22-23,25,29H,2,5-7,10-20H2,(H,35,36)/t22?,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50144532

((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...)Show SMILES OC(=O)[C@@H]1[C@@H](CCC2CCCNC2)C(=O)N1C(=O)N1CCN(CC1)C(=O)CCCCCc1ccccc1 Show InChI InChI=1S/C28H40N4O5/c33-24(12-6-2-5-10-21-8-3-1-4-9-21)30-16-18-31(19-17-30)28(37)32-25(27(35)36)23(26(32)34)14-13-22-11-7-15-29-20-22/h1,3-4,8-9,22-23,25,29H,2,5-7,10-20H2,(H,35,36)/t22?,23-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against tissue type plasminogen activator was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50220826
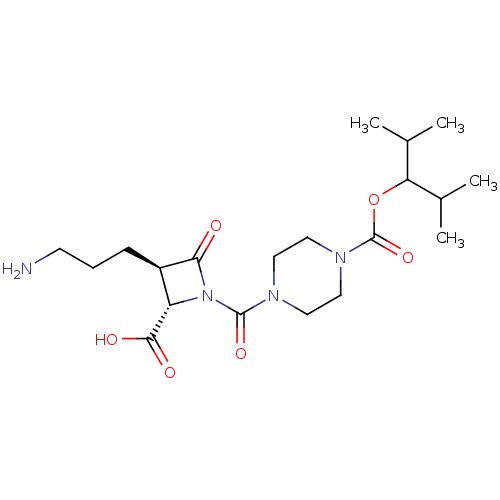
(CHEMBL303827)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CCCN)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C20H34N4O6/c1-12(2)16(13(3)4)30-20(29)23-10-8-22(9-11-23)19(28)24-15(18(26)27)14(17(24)25)6-5-7-21/h12-16H,5-11,21H2,1-4H3,(H,26,27)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against human tryptase was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144531

(4-((2S,3R)-2-Carboxy-4-oxo-3-piperidin-4-ylmethyl-...)Show SMILES CC(C)C(OC(=O)N1CCN(CC1)C(=O)N1[C@@H]([C@@H](CC2CCNCC2)C1=O)C(O)=O)C(C)C Show InChI InChI=1S/C23H38N4O6/c1-14(2)19(15(3)4)33-23(32)26-11-9-25(10-12-26)22(31)27-18(21(29)30)17(20(27)28)13-16-5-7-24-8-6-16/h14-19,24H,5-13H2,1-4H3,(H,29,30)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2227-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.011
BindingDB Entry DOI: 10.7270/Q2GM86Q3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data