

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144828 (5-(2,3,5,6-Tetrafluoro-benzenesulfonylamino)-[1,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146749 (2,3,5,6-Tetrafluoro-N-[3-methyl-5-sulfamoyl-3H-[1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144813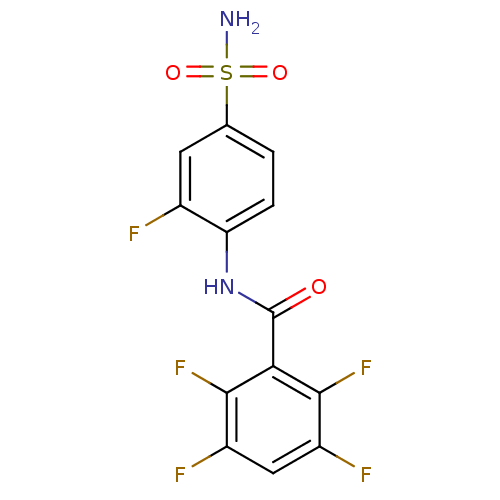 (2,3,5,6-Tetrafluoro-N-(2-fluoro-4-sulfamoyl-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144820 (2,3,5,6-Tetrafluoro-N-(5-sulfamoyl-[1,3,4]thiadiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146761 (5-(3-Pentafluorophenyl-ureido)-[1,3,4]thiadiazole-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146755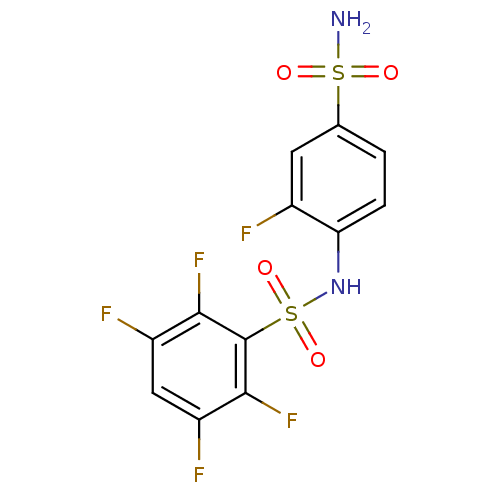 (2,3,5,6-Tetrafluoro-N-(2-fluoro-4-sulfamoyl-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146756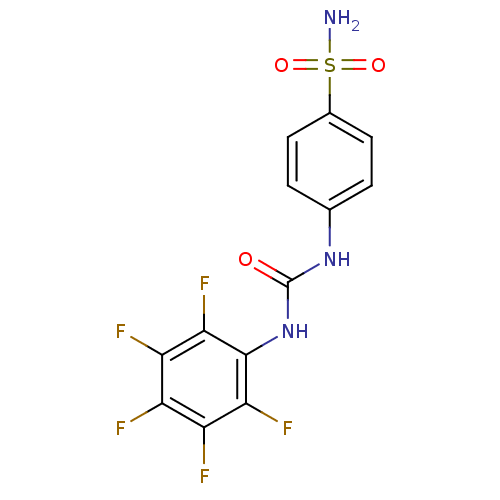 (4-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144822 (2,3,5,6-Tetrafluoro-N-[2-(4-sulfamoyl-phenyl)-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144826 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-benzyl)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144816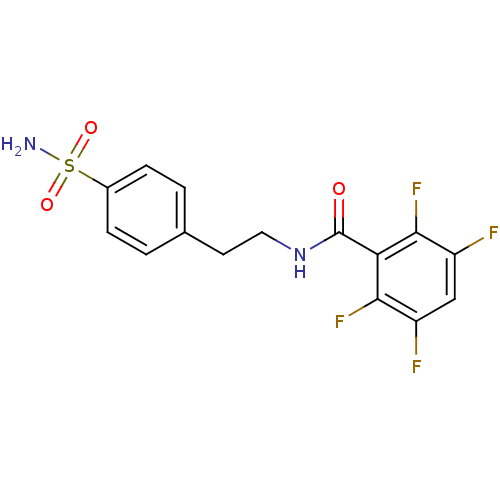 (2,3,5,6-Tetrafluoro-N-[2-(4-sulfamoyl-phenyl)-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144823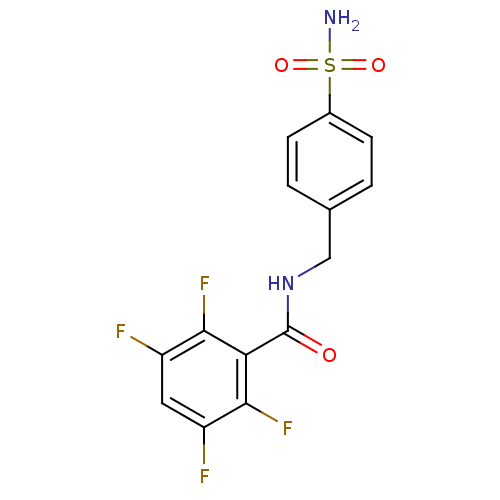 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-benzyl)-benzami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144828 (5-(2,3,5,6-Tetrafluoro-benzenesulfonylamino)-[1,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146753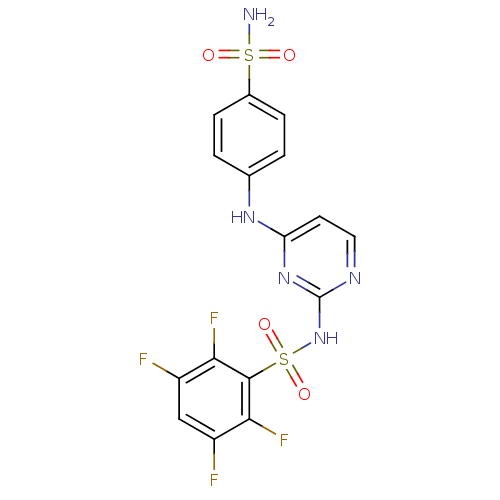 (2,3,5,6-Tetrafluoro-N-[4-(4-sulfamoyl-phenylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144818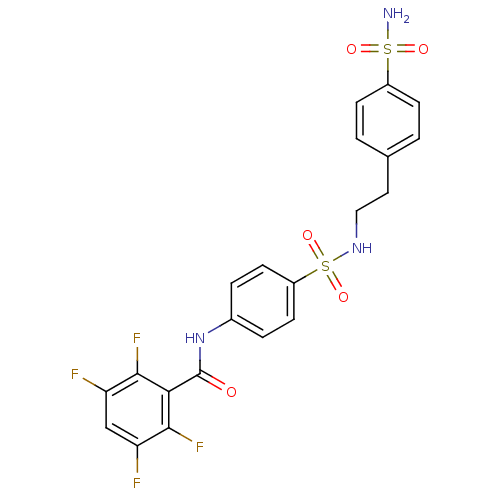 (2,3,5,6-Tetrafluoro-N-{4-[2-(4-sulfamoyl-phenyl)-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10881 (CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146750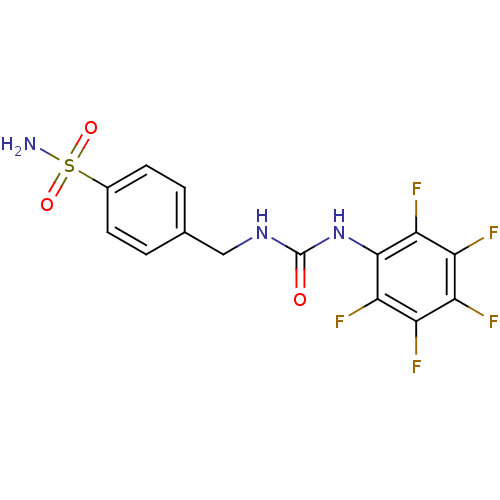 (4-(3-Pentafluorophenyl-ureidomethyl)-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146754 (4-[2-(3-Pentafluorophenyl-ureido)-ethyl]-benzenesu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144828 (5-(2,3,5,6-Tetrafluoro-benzenesulfonylamino)-[1,3,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144817 (2,3,5,6-Tetrafluoro-N-[4-(4-sulfamoyl-benzylsulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146758 (4-Chloro-6-(3-pentafluorophenyl-ureido)-benzene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144814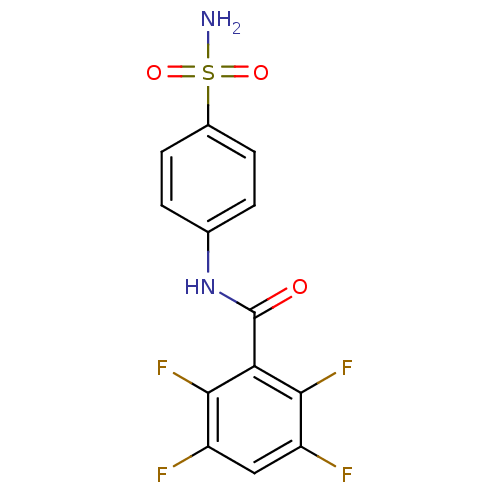 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-phenyl)-benzami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146748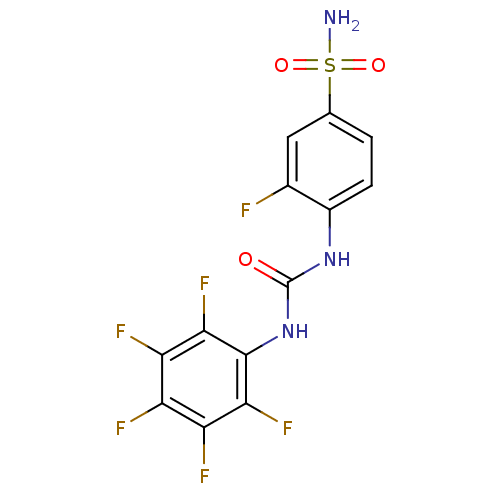 (3-Fluoro-4-(3-pentafluorophenyl-ureido)-benzenesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144824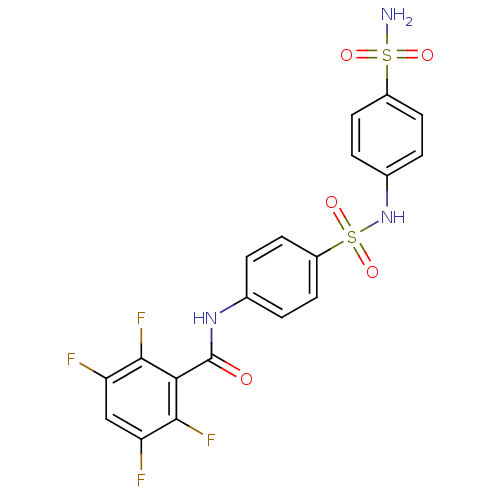 (2,3,5,6-Tetrafluoro-N-[4-(4-sulfamoyl-phenylsulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144819 (2,3,5,6-Tetrafluoro-N-(3-sulfamoyl-phenyl)-benzami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146756 (4-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146753 (2,3,5,6-Tetrafluoro-N-[4-(4-sulfamoyl-phenylamino)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144820 (2,3,5,6-Tetrafluoro-N-(5-sulfamoyl-[1,3,4]thiadiaz...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146751 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-phenyl)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146749 (2,3,5,6-Tetrafluoro-N-[3-methyl-5-sulfamoyl-3H-[1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146759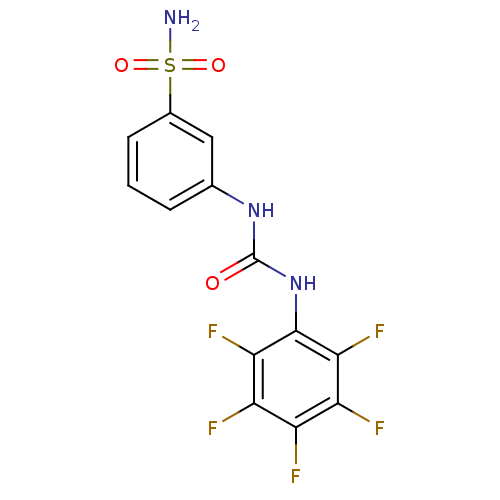 (3-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144813 (2,3,5,6-Tetrafluoro-N-(2-fluoro-4-sulfamoyl-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144815 (2,3,5,6-Tetrafluoro-N-(2-sulfamoyl-phenyl)-benzami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10883 (4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank MMDB PDB Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146754 (4-[2-(3-Pentafluorophenyl-ureido)-ethyl]-benzenesu...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146755 (2,3,5,6-Tetrafluoro-N-(2-fluoro-4-sulfamoyl-phenyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144827 (CHEMBL309592 | N-(2-Chloro-4-sulfamoyl-phenyl)-2,3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146757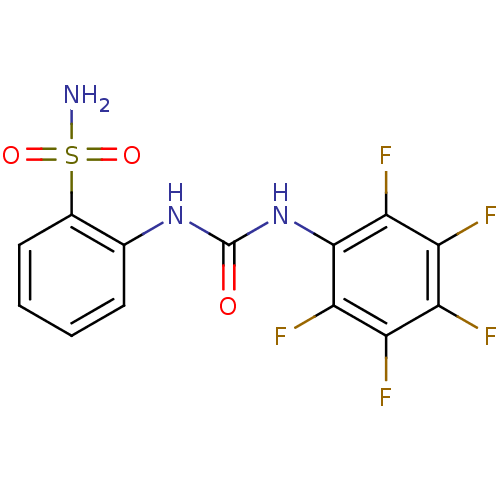 (2-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146761 (5-(3-Pentafluorophenyl-ureido)-[1,3,4]thiadiazole-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144818 (2,3,5,6-Tetrafluoro-N-{4-[2-(4-sulfamoyl-phenyl)-e...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146762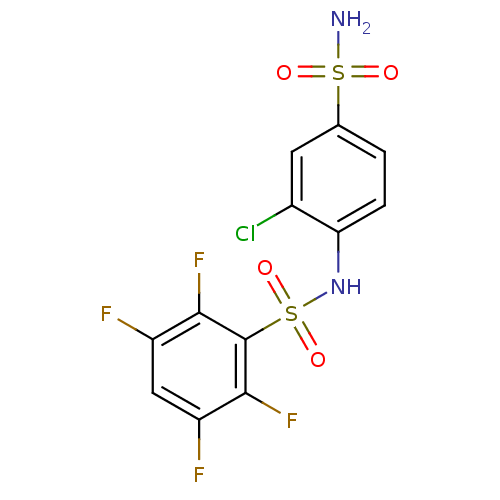 (CHEMBL317743 | N-(2-Chloro-4-sulfamoyl-phenyl)-2,3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144825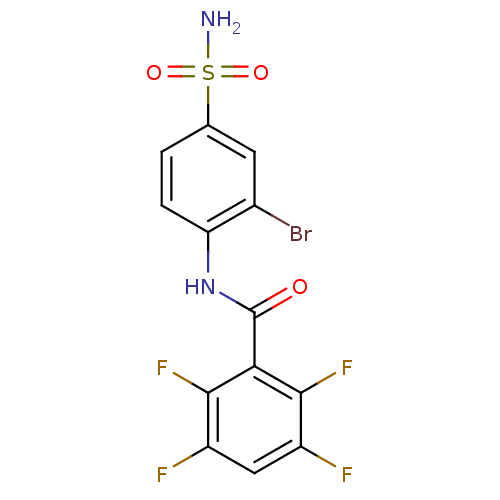 (CHEMBL75620 | N-(2-Bromo-4-sulfamoyl-phenyl)-2,3,5...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146750 (4-(3-Pentafluorophenyl-ureidomethyl)-benzenesulfon...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144817 (2,3,5,6-Tetrafluoro-N-[4-(4-sulfamoyl-benzylsulfam...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146758 (4-Chloro-6-(3-pentafluorophenyl-ureido)-benzene-1,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146762 (CHEMBL317743 | N-(2-Chloro-4-sulfamoyl-phenyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146748 (3-Fluoro-4-(3-pentafluorophenyl-ureido)-benzenesul...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144824 (2,3,5,6-Tetrafluoro-N-[4-(4-sulfamoyl-phenylsulfam...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144822 (2,3,5,6-Tetrafluoro-N-[2-(4-sulfamoyl-phenyl)-ethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144827 (CHEMBL309592 | N-(2-Chloro-4-sulfamoyl-phenyl)-2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146760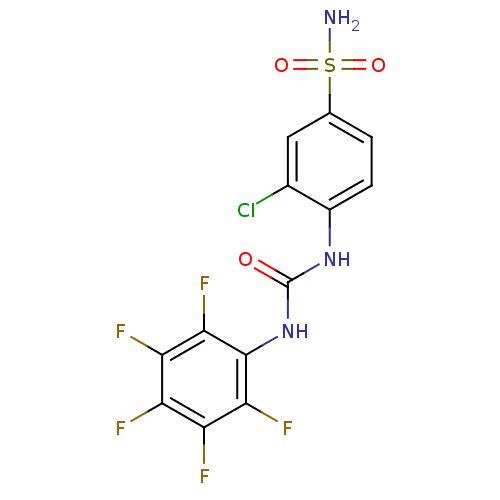 (3-Chloro-4-(3-pentafluorophenyl-ureido)-benzenesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50144825 (CHEMBL75620 | N-(2-Bromo-4-sulfamoyl-phenyl)-2,3,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144816 (2,3,5,6-Tetrafluoro-N-[2-(4-sulfamoyl-phenyl)-ethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50146752 (3-Bromo-4-(3-pentafluorophenyl-ureido)-benzenesulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase II (hCAII) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144823 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-benzyl)-benzami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144826 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-benzyl)-benzene...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM10881 (CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146760 (3-Chloro-4-(3-pentafluorophenyl-ureido)-benzenesul...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144820 (2,3,5,6-Tetrafluoro-N-(5-sulfamoyl-[1,3,4]thiadiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146749 (2,3,5,6-Tetrafluoro-N-[3-methyl-5-sulfamoyl-3H-[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146761 (5-(3-Pentafluorophenyl-ureido)-[1,3,4]thiadiazole-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146753 (2,3,5,6-Tetrafluoro-N-[4-(4-sulfamoyl-phenylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146751 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-phenyl)-benzene...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146752 (3-Bromo-4-(3-pentafluorophenyl-ureido)-benzenesulf...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM10883 (4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144814 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-phenyl)-benzami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144819 (2,3,5,6-Tetrafluoro-N-(3-sulfamoyl-phenyl)-benzami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146759 (3-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50146757 (2-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 485 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146755 (2,3,5,6-Tetrafluoro-N-(2-fluoro-4-sulfamoyl-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50144815 (2,3,5,6-Tetrafluoro-N-(2-sulfamoyl-phenyl)-benzami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against bovine carbonic anhydrase IV (CAIV) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146758 (4-Chloro-6-(3-pentafluorophenyl-ureido)-benzene-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144822 (2,3,5,6-Tetrafluoro-N-[2-(4-sulfamoyl-phenyl)-ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146748 (3-Fluoro-4-(3-pentafluorophenyl-ureido)-benzenesul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144813 (2,3,5,6-Tetrafluoro-N-(2-fluoro-4-sulfamoyl-phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10881 (CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144826 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-benzyl)-benzene...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144816 (2,3,5,6-Tetrafluoro-N-[2-(4-sulfamoyl-phenyl)-ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146762 (CHEMBL317743 | N-(2-Chloro-4-sulfamoyl-phenyl)-2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146754 (4-[2-(3-Pentafluorophenyl-ureido)-ethyl]-benzenesu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146756 (4-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144814 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-phenyl)-benzami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 975 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144823 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-benzyl)-benzami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146750 (4-(3-Pentafluorophenyl-ureidomethyl)-benzenesulfon...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144827 (CHEMBL309592 | N-(2-Chloro-4-sulfamoyl-phenyl)-2,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10883 (4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146760 (3-Chloro-4-(3-pentafluorophenyl-ureido)-benzenesul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146751 (2,3,5,6-Tetrafluoro-N-(4-sulfamoyl-phenyl)-benzene...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144815 (2,3,5,6-Tetrafluoro-N-(2-sulfamoyl-phenyl)-benzami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144819 (2,3,5,6-Tetrafluoro-N-(3-sulfamoyl-phenyl)-benzami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146757 (2-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146759 (3-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144825 (CHEMBL75620 | N-(2-Bromo-4-sulfamoyl-phenyl)-2,3,5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50146752 (3-Bromo-4-(3-pentafluorophenyl-ureido)-benzenesulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144818 (2,3,5,6-Tetrafluoro-N-{4-[2-(4-sulfamoyl-phenyl)-e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144817 (2,3,5,6-Tetrafluoro-N-[4-(4-sulfamoyl-benzylsulfam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50144824 (2,3,5,6-Tetrafluoro-N-[4-(4-sulfamoyl-phenylsulfam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10884 ((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description In vitro inhibitory activity against human carbonic anhydrase I (hCAI) | J Med Chem 47: 2796-804 (2004) Article DOI: 10.1021/jm031116j BindingDB Entry DOI: 10.7270/Q26974BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||