 Found 58 hits of Enzyme Inhibition Constant Data
Found 58 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
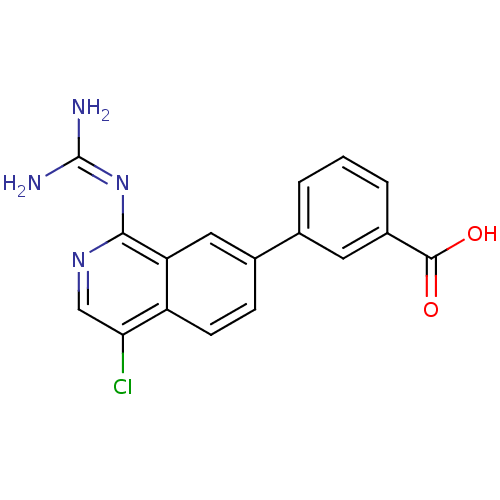
(Homo sapiens (Human)) | BDBM50147416

(3-(4-Chloro-1-guanidino-isoquinolin-7-yl)-4-methox...)Show SMILES [#6]-[#8]-c1ccc(cc1-c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1)-[#6](-[#8])=O Show InChI InChI=1S/C18H15ClN4O3/c1-26-15-5-3-10(17(24)25)7-12(15)9-2-4-11-13(6-9)16(23-18(20)21)22-8-14(11)19/h2-8H,1H3,(H,24,25)(H4,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
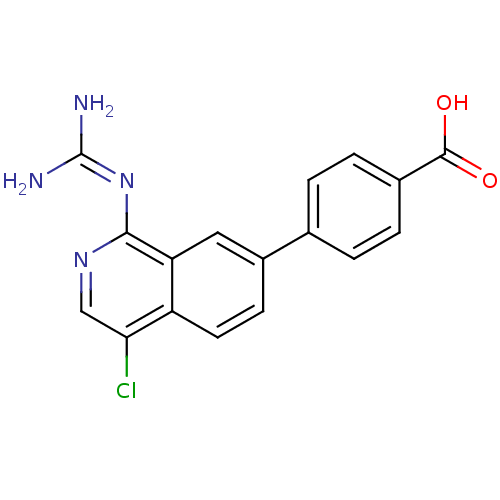
(Homo sapiens (Human)) | BDBM50147422
(3-(4-Chloro-1-guanidino-isoquinolin-7-yl)-benzoic ...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ncc(Cl)c2ccc(cc12)-c1cccc(c1)-[#6](-[#8])=O Show InChI InChI=1S/C17H13ClN4O2/c18-14-8-21-15(22-17(19)20)13-7-10(4-5-12(13)14)9-2-1-3-11(6-9)16(23)24/h1-8H,(H,23,24)(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147424
(4-(4-Chloro-1-guanidino-isoquinolin-7-yl)-benzoic ...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)-c1ccc(cc1)-[#6](-[#8])=O Show InChI InChI=1S/C17H13ClN4O2/c18-14-8-21-15(22-17(19)20)13-7-11(5-6-12(13)14)9-1-3-10(4-2-9)16(23)24/h1-8H,(H,23,24)(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147419
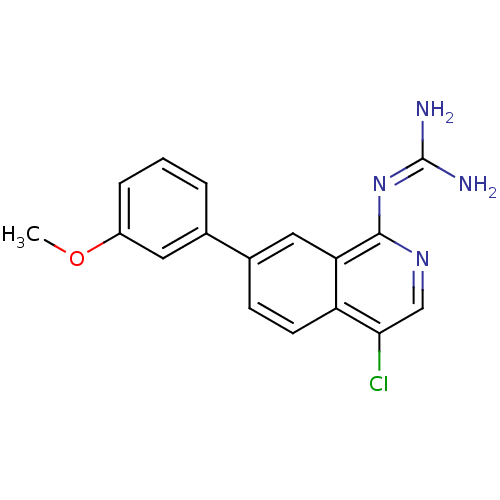
(CHEMBL320729 | N-[4-Chloro-7-(3-methoxy-phenyl)-is...)Show SMILES [#6]-[#8]-c1cccc(c1)-c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1 Show InChI InChI=1S/C17H15ClN4O/c1-23-12-4-2-3-10(7-12)11-5-6-13-14(8-11)16(22-17(19)20)21-9-15(13)18/h2-9H,1H3,(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147423

(CHEMBL432422 | N-(7-Benzo[1,3]dioxol-5-yl-4-chloro...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ncc(Cl)c2ccc(cc12)-c1ccc2-[#8]-[#6]-[#8]-c2c1 Show InChI InChI=1S/C17H13ClN4O2/c18-13-7-21-16(22-17(19)20)12-5-9(1-3-11(12)13)10-2-4-14-15(6-10)24-8-23-14/h1-7H,8H2,(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147418

(CHEMBL320493 | N-[4-Chloro-7-(2-methoxy-phenyl)-is...)Show SMILES [#6]-[#8]-c1ccccc1-c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1 Show InChI InChI=1S/C17H15ClN4O/c1-23-15-5-3-2-4-11(15)10-6-7-12-13(8-10)16(22-17(19)20)21-9-14(12)18/h2-9H,1H3,(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147415

(CHEMBL109274 | N-(4-Chloro-7-p-tolyl-isoquinolin-1...)Show SMILES [#6]-c1ccc(cc1)-c1ccc2c(Cl)cnc(\[#7]=[#6](\[#7])-[#7])c2c1 Show InChI InChI=1S/C17H15ClN4/c1-10-2-4-11(5-3-10)12-6-7-13-14(8-12)16(22-17(19)20)21-9-15(13)18/h2-9H,1H3,(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147409
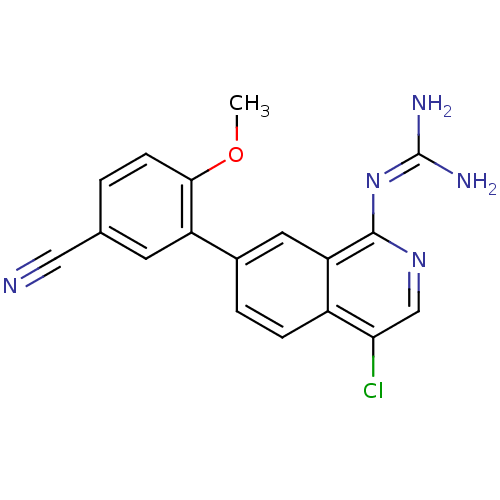
(CHEMBL324861 | N-[4-Chloro-7-(5-cyano-2-methoxy-ph...)Show SMILES [#6]-[#8]-c1ccc(cc1-c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1)C#N Show InChI InChI=1S/C18H14ClN5O/c1-25-16-5-2-10(8-20)6-13(16)11-3-4-12-14(7-11)17(24-18(21)22)23-9-15(12)19/h2-7,9H,1H3,(H4,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147404
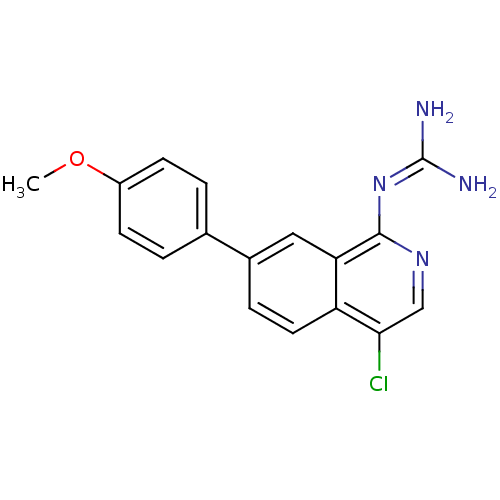
(CHEMBL109849 | N-[4-Chloro-7-(4-methoxy-phenyl)-is...)Show SMILES [#6]-[#8]-c1ccc(cc1)-c1ccc2c(Cl)cnc(\[#7]=[#6](\[#7])-[#7])c2c1 Show InChI InChI=1S/C17H15ClN4O/c1-23-12-5-2-10(3-6-12)11-4-7-13-14(8-11)16(22-17(19)20)21-9-15(13)18/h2-9H,1H3,(H4,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147417

(CHEMBL112785 | N-[4-Chloro-7-(3-cyano-phenyl)-isoq...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)-c1cccc(c1)C#N Show InChI InChI=1S/C17H12ClN5/c18-15-9-22-16(23-17(20)21)14-7-12(4-5-13(14)15)11-3-1-2-10(6-11)8-19/h1-7,9H,(H4,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147414
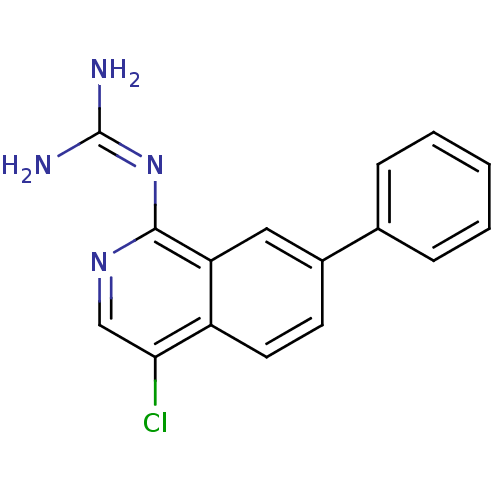
(CHEMBL109215 | N-(4-Chloro-7-phenyl-isoquinolin-1-...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ncc(Cl)c2ccc(cc12)-c1ccccc1 Show InChI InChI=1S/C16H13ClN4/c17-14-9-20-15(21-16(18)19)13-8-11(6-7-12(13)14)10-4-2-1-3-5-10/h1-9H,(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147429

(CHEMBL111452 | N-(7-Benzo[1,3]dioxol-5-yl-isoquino...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1nccc2ccc(cc12)-c1ccc2-[#8]-[#6]-[#8]-c2c1 Show InChI InChI=1S/C17H14N4O2/c18-17(19)21-16-13-7-11(2-1-10(13)5-6-20-16)12-3-4-14-15(8-12)23-9-22-14/h1-8H,9H2,(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147402
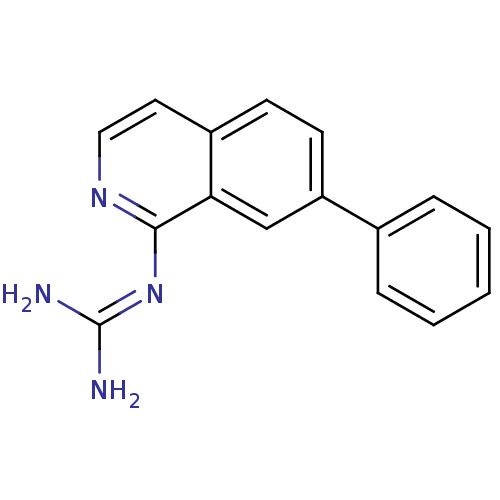
(CHEMBL323241 | N-(7-Phenyl-isoquinolin-1-yl)-guani...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1nccc2ccc(cc12)-c1ccccc1 Show InChI InChI=1S/C16H14N4/c17-16(18)20-15-14-10-13(11-4-2-1-3-5-11)7-6-12(14)8-9-19-15/h1-10H,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147413

(CHEMBL112786 | N-[4-Chloro-7-(4-cyano-phenyl)-isoq...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ncc(Cl)c2ccc(cc12)-c1ccc(cc1)C#N Show InChI InChI=1S/C17H12ClN5/c18-15-9-22-16(23-17(20)21)14-7-12(5-6-13(14)15)11-3-1-10(8-19)2-4-11/h1-7,9H,(H4,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147420

(CHEMBL323232 | N-(4-Bromo-isoquinolin-1-yl)-guanid...)Show InChI InChI=1S/C10H9BrN4/c11-8-5-14-9(15-10(12)13)7-4-2-1-3-6(7)8/h1-5H,(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147408
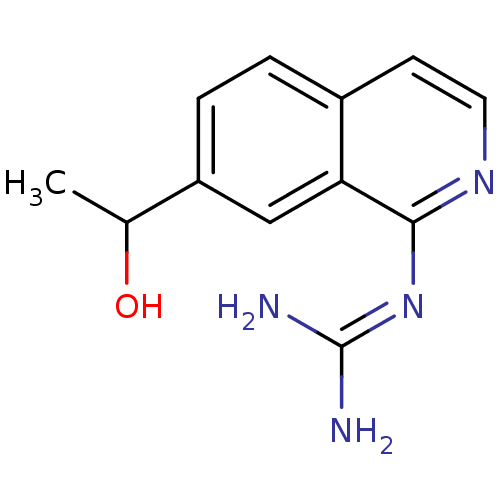
(CHEMBL113532 | N-[7-(1-Hydroxy-ethyl)-isoquinolin-...)Show SMILES [#6]-[#6](-[#8])-c1ccc2ccnc(\[#7]=[#6](\[#7])-[#7])c2c1 Show InChI InChI=1S/C12H14N4O/c1-7(17)9-3-2-8-4-5-15-11(10(8)6-9)16-12(13)14/h2-7,17H,1H3,(H4,13,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147407
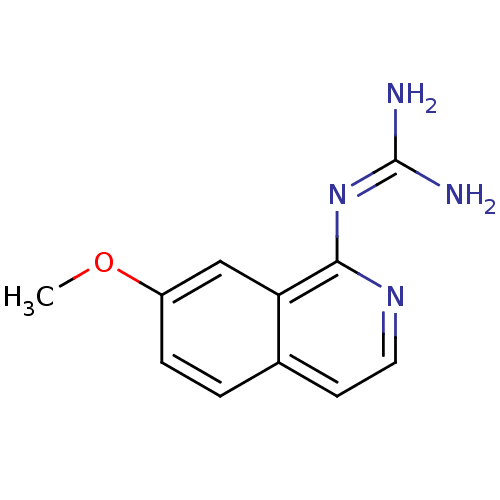
(CHEMBL110307 | N-(7-Methoxy-isoquinolin-1-yl)-guan...)Show SMILES [#6]-[#8]-c1ccc2ccnc(\[#7]=[#6](/[#7])-[#7])c2c1 Show InChI InChI=1S/C11H12N4O/c1-16-8-3-2-7-4-5-14-10(9(7)6-8)15-11(12)13/h2-6H,1H3,(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147406

(CHEMBL321848 | N-(7-Bromo-isoquinolin-1-yl)-guanid...)Show InChI InChI=1S/C10H9BrN4/c11-7-2-1-6-3-4-14-9(8(6)5-7)15-10(12)13/h1-5H,(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147410
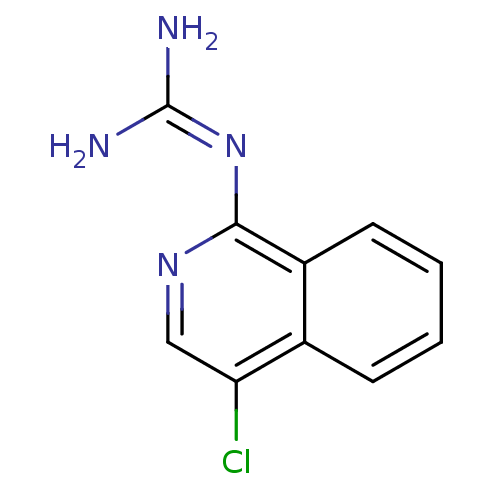
(CHEMBL326419 | N-(4-Chloro-isoquinolin-1-yl)-guani...)Show InChI InChI=1S/C10H9ClN4/c11-8-5-14-9(15-10(12)13)7-4-2-1-3-6(7)8/h1-5H,(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147427
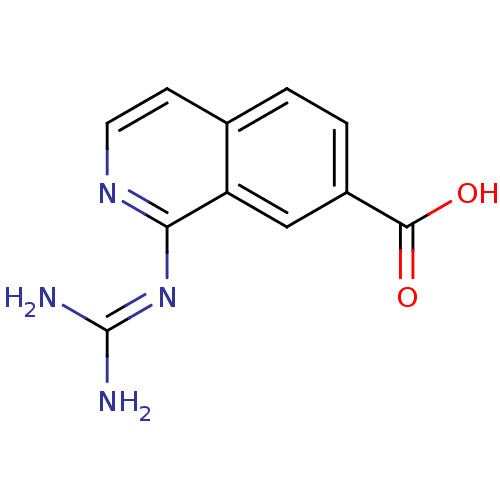
(1-Guanidino-isoquinoline-7-carboxylic acid | CHEMB...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1nccc2ccc(cc12)-[#6](-[#8])=O Show InChI InChI=1S/C11H10N4O2/c12-11(13)15-9-8-5-7(10(16)17)2-1-6(8)3-4-14-9/h1-5H,(H,16,17)(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147405
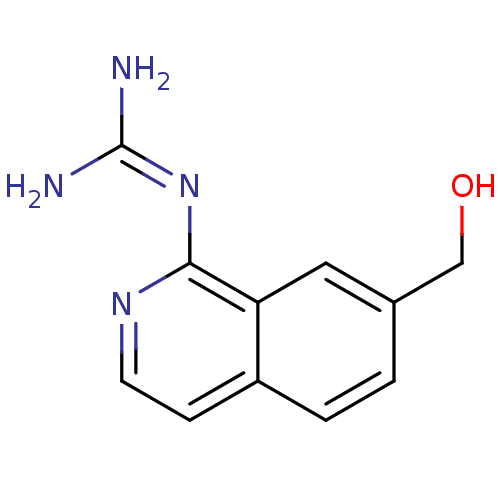
(CHEMBL324663 | N-(7-Hydroxymethyl-isoquinolin-1-yl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1nccc2ccc(-[#6]-[#8])cc12 Show InChI InChI=1S/C11H12N4O/c12-11(13)15-10-9-5-7(6-16)1-2-8(9)3-4-14-10/h1-5,16H,6H2,(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147431

(CHEMBL111343 | N-(4-Methyl-isoquinolin-1-yl)-guani...)Show SMILES [#6]-c1cnc(\[#7]=[#6](/[#7])-[#7])c2ccccc12 Show InChI InChI=1S/C11H12N4/c1-7-6-14-10(15-11(12)13)9-5-3-2-4-8(7)9/h2-6H,1H3,(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147412

(CHEMBL321822 | N-Isoquinolin-1-yl-guanidine)Show InChI InChI=1S/C10H10N4/c11-10(12)14-9-8-4-2-1-3-7(8)5-6-13-9/h1-6H,(H4,11,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147403

(CHEMBL107871 | N-(6-Bromo-isoquinolin-1-yl)-guanid...)Show InChI InChI=1S/C10H9BrN4/c11-7-1-2-8-6(5-7)3-4-14-9(8)15-10(12)13/h1-5H,(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147404

(CHEMBL109849 | N-[4-Chloro-7-(4-methoxy-phenyl)-is...)Show SMILES [#6]-[#8]-c1ccc(cc1)-c1ccc2c(Cl)cnc(\[#7]=[#6](\[#7])-[#7])c2c1 Show InChI InChI=1S/C17H15ClN4O/c1-23-12-5-2-10(3-6-12)11-4-7-13-14(8-11)16(22-17(19)20)21-9-15(13)18/h2-9H,1H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147423

(CHEMBL432422 | N-(7-Benzo[1,3]dioxol-5-yl-4-chloro...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1ncc(Cl)c2ccc(cc12)-c1ccc2-[#8]-[#6]-[#8]-c2c1 Show InChI InChI=1S/C17H13ClN4O2/c18-13-7-21-16(22-17(19)20)12-5-9(1-3-11(12)13)10-2-4-14-15(6-10)24-8-23-14/h1-7H,8H2,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147432
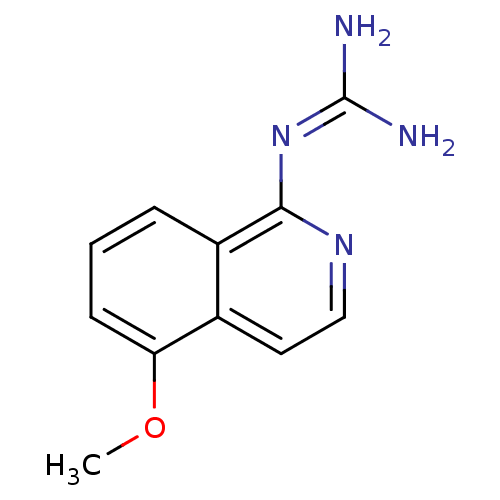
(CHEMBL322489 | N-(5-Methoxy-isoquinolin-1-yl)-guan...)Show SMILES [#6]-[#8]-c1cccc2c(\[#7]=[#6](/[#7])-[#7])nccc12 Show InChI InChI=1S/C11H12N4O/c1-16-9-4-2-3-8-7(9)5-6-14-10(8)15-11(12)13/h2-6H,1H3,(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147417

(CHEMBL112785 | N-[4-Chloro-7-(3-cyano-phenyl)-isoq...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ncc(Cl)c2ccc(cc12)-c1cccc(c1)C#N Show InChI InChI=1S/C17H12ClN5/c18-15-9-22-16(23-17(20)21)14-7-12(4-5-13(14)15)11-3-1-2-10(6-11)8-19/h1-7,9H,(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147416

(3-(4-Chloro-1-guanidino-isoquinolin-7-yl)-4-methox...)Show SMILES [#6]-[#8]-c1ccc(cc1-c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1)-[#6](-[#8])=O Show InChI InChI=1S/C18H15ClN4O3/c1-26-15-5-3-10(17(24)25)7-12(15)9-2-4-11-13(6-9)16(23-18(20)21)22-8-14(11)19/h2-8H,1H3,(H,24,25)(H4,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147425
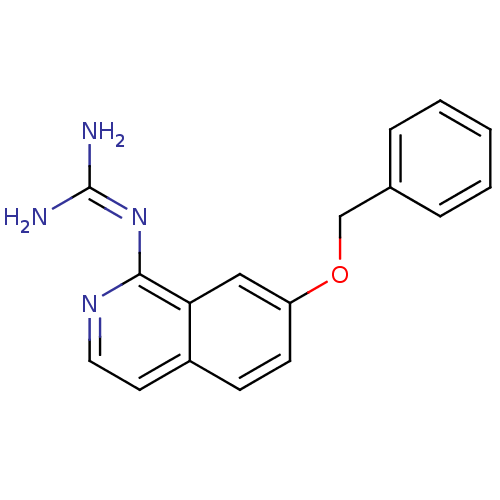
(CHEMBL107872 | N-(7-Benzyloxy-isoquinolin-1-yl)-gu...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1nccc2ccc(-[#8]-[#6]-c3ccccc3)cc12 Show InChI InChI=1S/C17H16N4O/c18-17(19)21-16-15-10-14(7-6-13(15)8-9-20-16)22-11-12-4-2-1-3-5-12/h1-10H,11H2,(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147411

(CHEMBL325094 | N-(5-Benzyloxy-isoquinolin-1-yl)-gu...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1nccc2c(-[#8]-[#6]-c3ccccc3)cccc12 Show InChI InChI=1S/C17H16N4O/c18-17(19)21-16-14-7-4-8-15(13(14)9-10-20-16)22-11-12-5-2-1-3-6-12/h1-10H,11H2,(H4,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
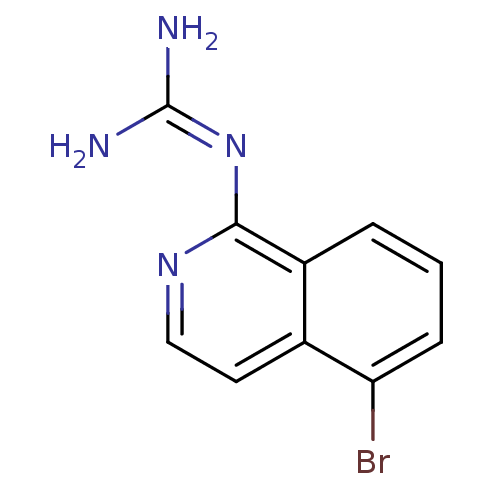
(Homo sapiens (Human)) | BDBM50147428
(CHEMBL110358 | N-(5-Bromo-isoquinolin-1-yl)-guanid...)Show InChI InChI=1S/C10H9BrN4/c11-8-3-1-2-7-6(8)4-5-14-9(7)15-10(12)13/h1-5H,(H4,12,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147415

(CHEMBL109274 | N-(4-Chloro-7-p-tolyl-isoquinolin-1...)Show SMILES [#6]-c1ccc(cc1)-c1ccc2c(Cl)cnc(\[#7]=[#6](\[#7])-[#7])c2c1 Show InChI InChI=1S/C17H15ClN4/c1-10-2-4-11(5-3-10)12-6-7-13-14(8-12)16(22-17(19)20)21-9-15(13)18/h2-9H,1H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147430
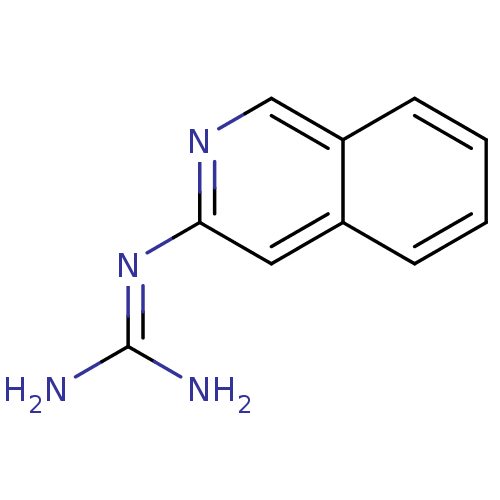
(CHEMBL109632 | N-Isoquinolin-3-yl-guanidine)Show InChI InChI=1S/C10H10N4/c11-10(12)14-9-5-7-3-1-2-4-8(7)6-13-9/h1-6H,(H4,11,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147429

(CHEMBL111452 | N-(7-Benzo[1,3]dioxol-5-yl-isoquino...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1nccc2ccc(cc12)-c1ccc2-[#8]-[#6]-[#8]-c2c1 Show InChI InChI=1S/C17H14N4O2/c18-17(19)21-16-13-7-11(2-1-10(13)5-6-20-16)12-3-4-14-15(8-12)23-9-22-14/h1-8H,9H2,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147425

(CHEMBL107872 | N-(7-Benzyloxy-isoquinolin-1-yl)-gu...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1nccc2ccc(-[#8]-[#6]-c3ccccc3)cc12 Show InChI InChI=1S/C17H16N4O/c18-17(19)21-16-15-10-14(7-6-13(15)8-9-20-16)22-11-12-4-2-1-3-5-12/h1-10H,11H2,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147411

(CHEMBL325094 | N-(5-Benzyloxy-isoquinolin-1-yl)-gu...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1nccc2c(-[#8]-[#6]-c3ccccc3)cccc12 Show InChI InChI=1S/C17H16N4O/c18-17(19)21-16-14-7-4-8-15(13(14)9-10-20-16)22-11-12-5-2-1-3-6-12/h1-10H,11H2,(H4,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147418

(CHEMBL320493 | N-[4-Chloro-7-(2-methoxy-phenyl)-is...)Show SMILES [#6]-[#8]-c1ccccc1-c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1 Show InChI InChI=1S/C17H15ClN4O/c1-23-15-5-3-2-4-11(15)10-6-7-12-13(8-10)16(22-17(19)20)21-9-14(12)18/h2-9H,1H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147418

(CHEMBL320493 | N-[4-Chloro-7-(2-methoxy-phenyl)-is...)Show SMILES [#6]-[#8]-c1ccccc1-c1ccc2c(Cl)cnc(\[#7]=[#6](/[#7])-[#7])c2c1 Show InChI InChI=1S/C17H15ClN4O/c1-23-15-5-3-2-4-11(15)10-6-7-12-13(8-10)16(22-17(19)20)21-9-14(12)18/h2-9H,1H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147415

(CHEMBL109274 | N-(4-Chloro-7-p-tolyl-isoquinolin-1...)Show SMILES [#6]-c1ccc(cc1)-c1ccc2c(Cl)cnc(\[#7]=[#6](\[#7])-[#7])c2c1 Show InChI InChI=1S/C17H15ClN4/c1-10-2-4-11(5-3-10)12-6-7-13-14(8-12)16(22-17(19)20)21-9-15(13)18/h2-9H,1H3,(H4,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147408

(CHEMBL113532 | N-[7-(1-Hydroxy-ethyl)-isoquinolin-...)Show SMILES [#6]-[#6](-[#8])-c1ccc2ccnc(\[#7]=[#6](\[#7])-[#7])c2c1 Show InChI InChI=1S/C12H14N4O/c1-7(17)9-3-2-8-4-5-15-11(10(8)6-9)16-12(13)14/h2-7,17H,1H3,(H4,13,14,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50053618
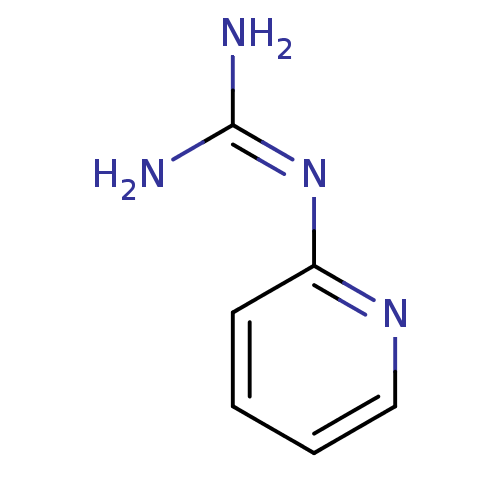
(CHEMBL128047 | N-Pyridin-2-yl-guanidine)Show InChI InChI=1S/C6H8N4/c7-6(8)10-5-3-1-2-4-9-5/h1-4H,(H4,7,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147402

(CHEMBL323241 | N-(7-Phenyl-isoquinolin-1-yl)-guani...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1nccc2ccc(cc12)-c1ccccc1 Show InChI InChI=1S/C16H14N4/c17-16(18)20-15-14-10-13(11-4-2-1-3-5-11)7-6-12(14)8-9-19-15/h1-10H,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147426

(CHEMBL111036 | N-(5-Phenyl-isoquinolin-1-yl)-guani...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1nccc2c(cccc12)-c1ccccc1 Show InChI InChI=1S/C16H14N4/c17-16(18)20-15-14-8-4-7-12(13(14)9-10-19-15)11-5-2-1-3-6-11/h1-10H,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147406

(CHEMBL321848 | N-(7-Bromo-isoquinolin-1-yl)-guanid...)Show InChI InChI=1S/C10H9BrN4/c11-7-2-1-6-3-4-14-9(8(6)5-7)15-10(12)13/h1-5H,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147412

(CHEMBL321822 | N-Isoquinolin-1-yl-guanidine)Show InChI InChI=1S/C10H10N4/c11-10(12)14-9-8-4-2-1-3-7(8)5-6-13-9/h1-6H,(H4,11,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasmin was evaluated using chromozym-PL as substrate at 1 mM |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147406

(CHEMBL321848 | N-(7-Bromo-isoquinolin-1-yl)-guanid...)Show InChI InChI=1S/C10H9BrN4/c11-7-2-1-6-3-4-14-9(8(6)5-7)15-10(12)13/h1-5H,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147426

(CHEMBL111036 | N-(5-Phenyl-isoquinolin-1-yl)-guani...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1nccc2c(cccc12)-c1ccccc1 Show InChI InChI=1S/C16H14N4/c17-16(18)20-15-14-8-4-7-12(13(14)9-10-19-15)11-5-2-1-3-6-11/h1-10H,(H4,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147426

(CHEMBL111036 | N-(5-Phenyl-isoquinolin-1-yl)-guani...)Show SMILES [#7]\[#6](-[#7])=[#7]\c1nccc2c(cccc12)-c1ccccc1 Show InChI InChI=1S/C16H14N4/c17-16(18)20-15-14-8-4-7-12(13(14)9-10-19-15)11-5-2-1-3-6-11/h1-10H,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147402

(CHEMBL323241 | N-(7-Phenyl-isoquinolin-1-yl)-guani...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1nccc2ccc(cc12)-c1ccccc1 Show InChI InChI=1S/C16H14N4/c17-16(18)20-15-14-10-13(11-4-2-1-3-5-11)7-6-12(14)8-9-19-15/h1-10H,(H4,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147412

(CHEMBL321822 | N-Isoquinolin-1-yl-guanidine)Show InChI InChI=1S/C10H10N4/c11-10(12)14-9-8-4-2-1-3-7(8)5-6-13-9/h1-6H,(H4,11,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147410

(CHEMBL326419 | N-(4-Chloro-isoquinolin-1-yl)-guani...)Show InChI InChI=1S/C10H9ClN4/c11-8-5-14-9(15-10(12)13)7-4-2-1-3-6(7)8/h1-5H,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147403

(CHEMBL107871 | N-(6-Bromo-isoquinolin-1-yl)-guanid...)Show InChI InChI=1S/C10H9BrN4/c11-7-1-2-8-6(5-7)3-4-14-9(8)15-10(12)13/h1-5H,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147428

(CHEMBL110358 | N-(5-Bromo-isoquinolin-1-yl)-guanid...)Show InChI InChI=1S/C10H9BrN4/c11-8-3-1-2-7-6(8)4-5-14-9(7)15-10(12)13/h1-5H,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147420

(CHEMBL323232 | N-(4-Bromo-isoquinolin-1-yl)-guanid...)Show InChI InChI=1S/C10H9BrN4/c11-8-5-14-9(15-10(12)13)7-4-2-1-3-6(7)8/h1-5H,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147421
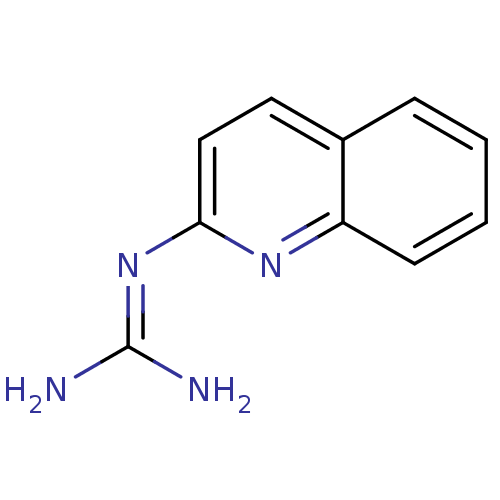
(CHEMBL109751 | N-Quinolin-2-yl-guanidine)Show InChI InChI=1S/C10H10N4/c11-10(12)14-9-6-5-7-3-1-2-4-8(7)13-9/h1-6H,(H4,11,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human urokinase-type plasminogen activator was evaluated using S-2444 as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50147428

(CHEMBL110358 | N-(5-Bromo-isoquinolin-1-yl)-guanid...)Show InChI InChI=1S/C10H9BrN4/c11-8-3-1-2-7-6(8)4-5-14-9(7)15-10(12)13/h1-5H,(H4,12,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin activity with chromozym-PL as substrate |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50147421

(CHEMBL109751 | N-Quinolin-2-yl-guanidine)Show InChI InChI=1S/C10H10N4/c11-10(12)14-9-6-5-7-3-1-2-4-8(7)13-9/h1-6H,(H4,11,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human tissue type plasminogen activator using S-2444 |
Bioorg Med Chem Lett 14: 3227-30 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.094
BindingDB Entry DOI: 10.7270/Q2125S3C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data