 Found 59 hits of Enzyme Inhibition Constant Data
Found 59 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147472

(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCO)c2ncccc12 |t:9| Show InChI InChI=1S/C21H19N3O4/c1-28-16-8-3-2-6-14(16)17-18(21(27)23-20(17)26)15-12-24(10-5-11-25)19-13(15)7-4-9-22-19/h2-4,6-9,12,25H,5,10-11H2,1H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147465
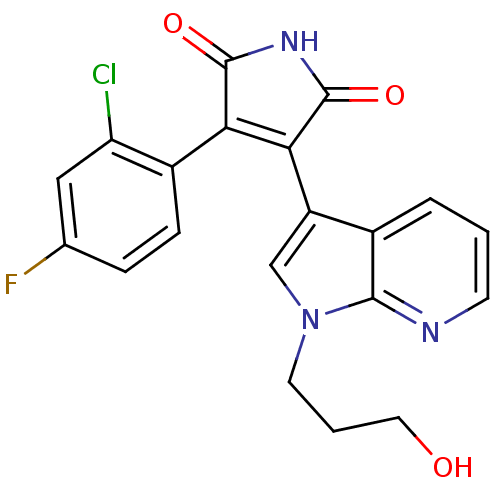
(3-(2-Chloro-4-fluoro-phenyl)-4-[1-(3-hydroxy-propy...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccc(F)cc2Cl)c2cccnc12 |t:7| Show InChI InChI=1S/C20H15ClFN3O3/c21-15-9-11(22)4-5-13(15)16-17(20(28)24-19(16)27)14-10-25(7-2-8-26)18-12(14)3-1-6-23-18/h1,3-6,9-10,26H,2,7-8H2,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147458
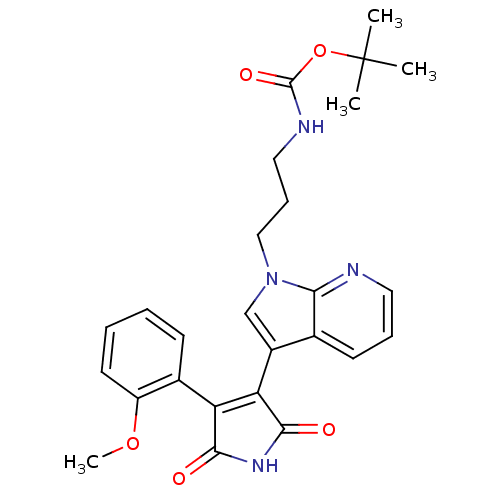
((3-{3-[4-(2-Methoxy-phenyl)-2,5-dioxo-2,5-dihydro-...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC(=O)OC(C)(C)C)c2ncccc12 |t:9| Show InChI InChI=1S/C26H28N4O5/c1-26(2,3)35-25(33)28-13-8-14-30-15-18(16-10-7-12-27-22(16)30)21-20(23(31)29-24(21)32)17-9-5-6-11-19(17)34-4/h5-7,9-12,15H,8,13-14H2,1-4H3,(H,28,33)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147464

(CHEMBL109977 | N-(3-{3-[4-(2-Methoxy-phenyl)-2,5-d...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC=O)c2ncccc12 |t:9| Show InChI InChI=1S/C22H20N4O4/c1-30-17-8-3-2-6-15(17)18-19(22(29)25-21(18)28)16-12-26(11-5-9-23-13-27)20-14(16)7-4-10-24-20/h2-4,6-8,10,12-13H,5,9,11H2,1H3,(H,23,27)(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147473
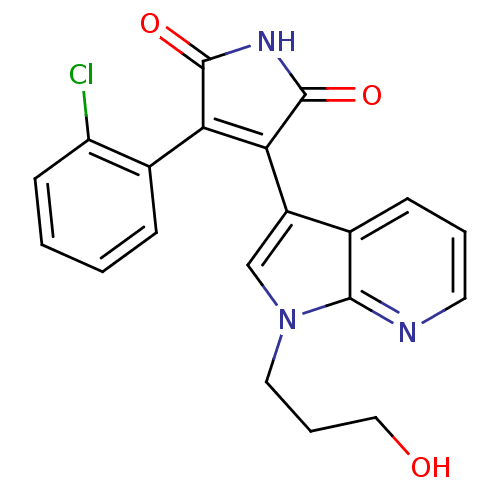
(3-(2-Chloro-phenyl)-4-[1-(3-hydroxy-propyl)-1H-pyr...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2cccnc12 |t:7| Show InChI InChI=1S/C20H16ClN3O3/c21-15-7-2-1-5-13(15)16-17(20(27)23-19(16)26)14-11-24(9-4-10-25)18-12(14)6-3-8-22-18/h1-3,5-8,11,25H,4,9-10H2,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147461
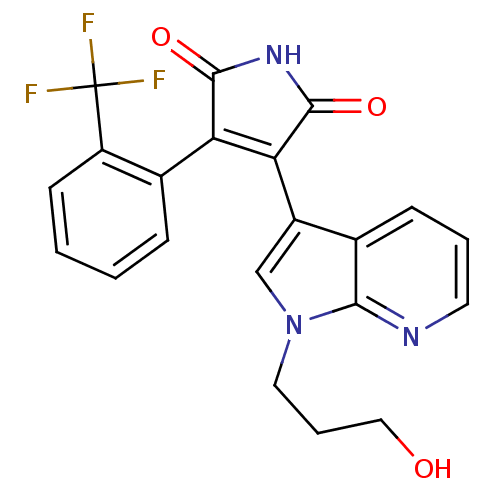
(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2C(F)(F)F)c2cccnc12 |t:7| Show InChI InChI=1S/C21H16F3N3O3/c22-21(23,24)15-7-2-1-5-13(15)16-17(20(30)26-19(16)29)14-11-27(9-4-10-28)18-12(14)6-3-8-25-18/h1-3,5-8,11,28H,4,9-10H2,(H,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147459
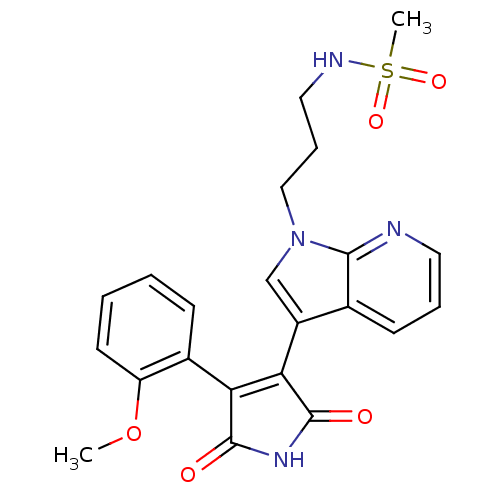
(CHEMBL326208 | N-(3-{3-[4-(2-Methoxy-phenyl)-2,5-d...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNS(C)(=O)=O)c2ncccc12 |t:9| Show InChI InChI=1S/C22H22N4O5S/c1-31-17-9-4-3-7-15(17)18-19(22(28)25-21(18)27)16-13-26(12-6-11-24-32(2,29)30)20-14(16)8-5-10-23-20/h3-5,7-10,13,24H,6,11-12H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147462
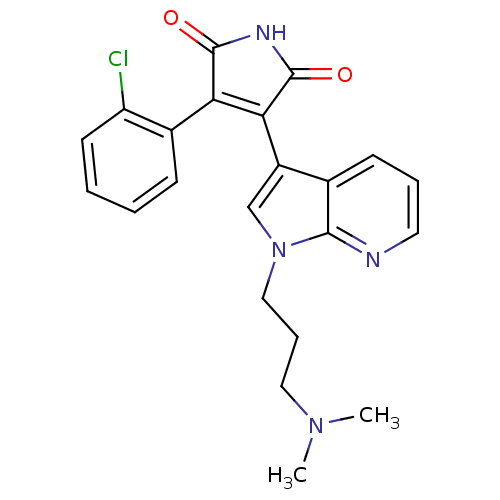
(3-(2-Chloro-phenyl)-4-[1-(3-dimethylamino-propyl)-...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2cccnc12 |t:9| Show InChI InChI=1S/C22H21ClN4O2/c1-26(2)11-6-12-27-13-16(14-8-5-10-24-20(14)27)19-18(21(28)25-22(19)29)15-7-3-4-9-17(15)23/h3-5,7-10,13H,6,11-12H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147468

(3-(7-Azaindolyl)-4-arylmaleimide analogue | CHEMBL...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNS(N)(=O)=O)c2ncccc12 |t:9| Show InChI InChI=1S/C21H21N5O5S/c1-31-16-8-3-2-6-14(16)17-18(21(28)25-20(17)27)15-12-26(11-5-10-24-32(22,29)30)19-13(15)7-4-9-23-19/h2-4,6-9,12,24H,5,10-11H2,1H3,(H2,22,29,30)(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147463
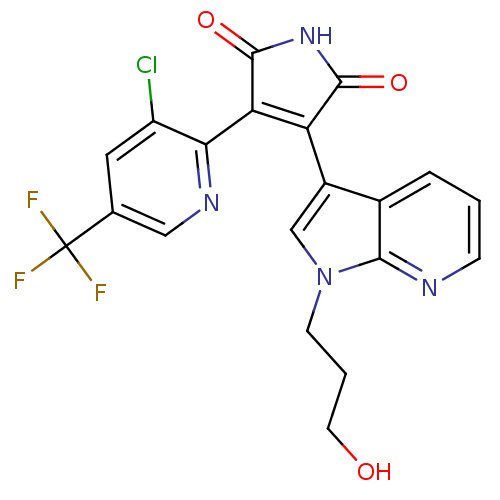
(3-(3-Chloro-5-trifluoromethyl-pyridin-2-yl)-4-[1-(...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ncc(cc2Cl)C(F)(F)F)c2cccnc12 |t:7| Show InChI InChI=1S/C20H14ClF3N4O3/c21-13-7-10(20(22,23)24)8-26-16(13)15-14(18(30)27-19(15)31)12-9-28(5-2-6-29)17-11(12)3-1-4-25-17/h1,3-4,7-9,29H,2,5-6H2,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147471
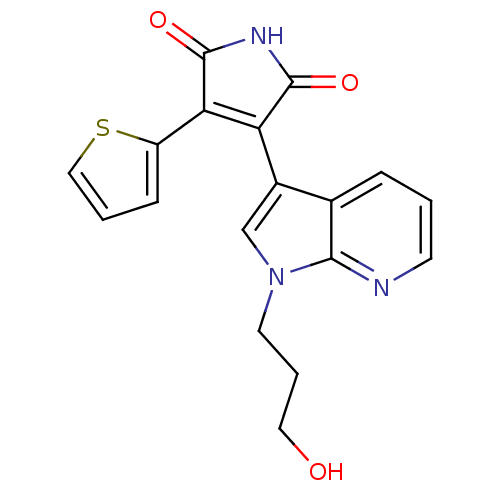
(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2cccs2)c2cccnc12 |t:7| Show InChI InChI=1S/C18H15N3O3S/c22-8-3-7-21-10-12(11-4-1-6-19-16(11)21)14-15(13-5-2-9-25-13)18(24)20-17(14)23/h1-2,4-6,9-10,22H,3,7-8H2,(H,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147469

(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2cccc3ccccc23)c2cccnc12 |t:7| Show InChI InChI=1S/C24H19N3O3/c28-13-5-12-27-14-19(18-10-4-11-25-22(18)27)21-20(23(29)26-24(21)30)17-9-3-7-15-6-1-2-8-16(15)17/h1-4,6-11,14,28H,5,12-13H2,(H,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147460
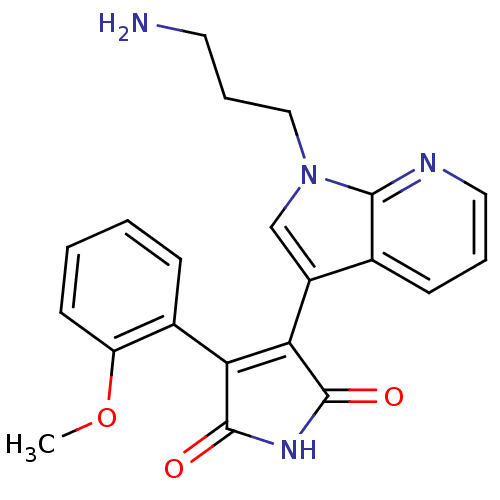
(3-[1-(3-Amino-propyl)-1H-pyrrolo[2,3-b]pyridin-3-y...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCN)c2ncccc12 |t:9| Show InChI InChI=1S/C21H20N4O3/c1-28-16-8-3-2-6-14(16)17-18(21(27)24-20(17)26)15-12-25(11-5-9-22)19-13(15)7-4-10-23-19/h2-4,6-8,10,12H,5,9,11,22H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147457
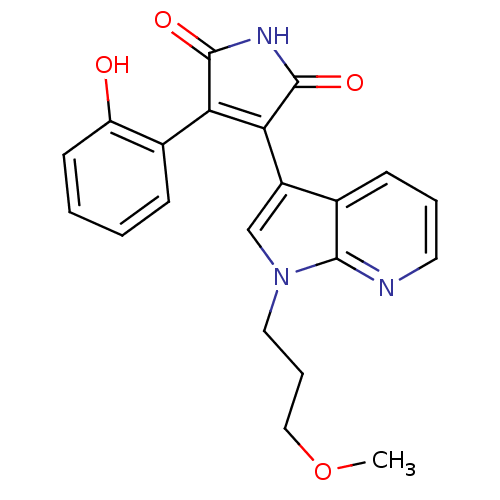
(3-(2-Hydroxy-phenyl)-4-[1-(3-hydroxy-propyl)-1H-py...)Show SMILES COCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2O)c2cccnc12 |t:8| Show InChI InChI=1S/C21H19N3O4/c1-28-11-5-10-24-12-15(13-7-4-9-22-19(13)24)18-17(20(26)23-21(18)27)14-6-2-3-8-16(14)25/h2-4,6-9,12,25H,5,10-11H2,1H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147466

(3-(1-Ethyl-1H-pyrrolo[2,3-b]pyridin-3-yl)-4-[1-(3-...)Show SMILES CCn1cc(C2=C(C(=O)NC2=O)c2cn(CCCO)c3ncccc23)c2cccnc12 |t:5| Show InChI InChI=1S/C23H21N5O3/c1-2-27-12-16(14-6-3-8-24-20(14)27)18-19(23(31)26-22(18)30)17-13-28(10-5-11-29)21-15(17)7-4-9-25-21/h3-4,6-9,12-13,29H,2,5,10-11H2,1H3,(H,26,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50147458

((3-{3-[4-(2-Methoxy-phenyl)-2,5-dioxo-2,5-dihydro-...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC(=O)OC(C)(C)C)c2ncccc12 |t:9| Show InChI InChI=1S/C26H28N4O5/c1-26(2,3)35-25(33)28-13-8-14-30-15-18(16-10-7-12-27-22(16)30)21-20(23(31)29-24(21)32)17-9-5-6-11-19(17)34-4/h5-7,9-12,15H,8,13-14H2,1-4H3,(H,28,33)(H,29,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 2-cyclin A |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147467

(3-[1-(3-Dimethylamino-propyl)-1H-pyrrolo[2,3-b]pyr...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2cccc3ccccc23)c2cccnc12 |t:9| Show InChI InChI=1S/C26H24N4O2/c1-29(2)14-7-15-30-16-21(20-12-6-13-27-24(20)30)23-22(25(31)28-26(23)32)19-11-5-9-17-8-3-4-10-18(17)19/h3-6,8-13,16H,7,14-15H2,1-2H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3
(Homo sapiens (Human)) | BDBM50147458

((3-{3-[4-(2-Methoxy-phenyl)-2,5-dioxo-2,5-dihydro-...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC(=O)OC(C)(C)C)c2ncccc12 |t:9| Show InChI InChI=1S/C26H28N4O5/c1-26(2,3)35-25(33)28-13-8-14-30-15-18(16-10-7-12-27-22(16)30)21-20(23(31)29-24(21)32)17-9-5-6-11-19(17)34-4/h5-7,9-12,15H,8,13-14H2,1-4H3,(H,28,33)(H,29,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 1-cyclin B |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147467

(3-[1-(3-Dimethylamino-propyl)-1H-pyrrolo[2,3-b]pyr...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2cccc3ccccc23)c2cccnc12 |t:9| Show InChI InChI=1S/C26H24N4O2/c1-29(2)14-7-15-30-16-21(20-12-6-13-27-24(20)30)23-22(25(31)28-26(23)32)19-11-5-9-17-8-3-4-10-18(17)19/h3-6,8-13,16H,7,14-15H2,1-2H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147469

(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2cccc3ccccc23)c2cccnc12 |t:7| Show InChI InChI=1S/C24H19N3O3/c28-13-5-12-27-14-19(18-10-4-11-25-22(18)27)21-20(23(29)26-24(21)30)17-9-3-7-15-6-1-2-8-16(15)17/h1-4,6-11,14,28H,5,12-13H2,(H,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50147457

(3-(2-Hydroxy-phenyl)-4-[1-(3-hydroxy-propyl)-1H-py...)Show SMILES COCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2O)c2cccnc12 |t:8| Show InChI InChI=1S/C21H19N3O4/c1-28-11-5-10-24-12-15(13-7-4-9-22-19(13)24)18-17(20(26)23-21(18)27)14-6-2-3-8-16(14)25/h2-4,6-9,12,25H,5,10-11H2,1H3,(H,23,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 2-cyclin A |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147470
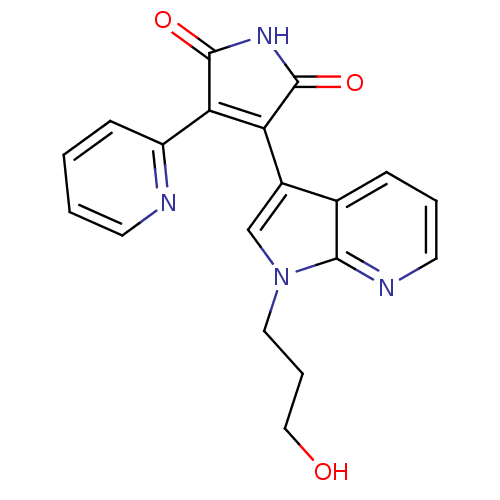
(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccccn2)c2cccnc12 |t:7| Show InChI InChI=1S/C19H16N4O3/c24-10-4-9-23-11-13(12-5-3-8-21-17(12)23)15-16(19(26)22-18(15)25)14-6-1-2-7-20-14/h1-3,5-8,11,24H,4,9-10H2,(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rabbit glycogen synthase kinase-3 beta using protein phosphatase inhibitor-2 as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50147458

((3-{3-[4-(2-Methoxy-phenyl)-2,5-dioxo-2,5-dihydro-...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC(=O)OC(C)(C)C)c2ncccc12 |t:9| Show InChI InChI=1S/C26H28N4O5/c1-26(2,3)35-25(33)28-13-8-14-30-15-18(16-10-7-12-27-22(16)30)21-20(23(31)29-24(21)32)17-9-5-6-11-19(17)34-4/h5-7,9-12,15H,8,13-14H2,1-4H3,(H,28,33)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50147458

((3-{3-[4-(2-Methoxy-phenyl)-2,5-dioxo-2,5-dihydro-...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC(=O)OC(C)(C)C)c2ncccc12 |t:9| Show InChI InChI=1S/C26H28N4O5/c1-26(2,3)35-25(33)28-13-8-14-30-15-18(16-10-7-12-27-22(16)30)21-20(23(31)29-24(21)32)17-9-5-6-11-19(17)34-4/h5-7,9-12,15H,8,13-14H2,1-4H3,(H,28,33)(H,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Ribosomal S6 kinase 3 |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3
(Homo sapiens (Human)) | BDBM50147457

(3-(2-Hydroxy-phenyl)-4-[1-(3-hydroxy-propyl)-1H-py...)Show SMILES COCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2O)c2cccnc12 |t:8| Show InChI InChI=1S/C21H19N3O4/c1-28-11-5-10-24-12-15(13-7-4-9-22-19(13)24)18-17(20(26)23-21(18)27)14-6-2-3-8-16(14)25/h2-4,6-9,12,25H,5,10-11H2,1H3,(H,23,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 1-cyclin B |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147473

(3-(2-Chloro-phenyl)-4-[1-(3-hydroxy-propyl)-1H-pyr...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2cccnc12 |t:7| Show InChI InChI=1S/C20H16ClN3O3/c21-15-7-2-1-5-13(15)16-17(20(27)23-19(16)26)14-11-24(9-4-10-25)18-12(14)6-3-8-22-18/h1-3,5-8,11,25H,4,9-10H2,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147468

(3-(7-Azaindolyl)-4-arylmaleimide analogue | CHEMBL...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNS(N)(=O)=O)c2ncccc12 |t:9| Show InChI InChI=1S/C21H21N5O5S/c1-31-16-8-3-2-6-14(16)17-18(21(28)25-20(17)27)15-12-26(11-5-10-24-32(22,29)30)19-13(15)7-4-9-23-19/h2-4,6-9,12,24H,5,10-11H2,1H3,(H2,22,29,30)(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147466

(3-(1-Ethyl-1H-pyrrolo[2,3-b]pyridin-3-yl)-4-[1-(3-...)Show SMILES CCn1cc(C2=C(C(=O)NC2=O)c2cn(CCCO)c3ncccc23)c2cccnc12 |t:5| Show InChI InChI=1S/C23H21N5O3/c1-2-27-12-16(14-6-3-8-24-20(14)27)18-19(23(31)26-22(18)30)17-13-28(10-5-11-29)21-15(17)7-4-9-25-21/h3-4,6-9,12-13,29H,2,5,10-11H2,1H3,(H,26,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147460

(3-[1-(3-Amino-propyl)-1H-pyrrolo[2,3-b]pyridin-3-y...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCN)c2ncccc12 |t:9| Show InChI InChI=1S/C21H20N4O3/c1-28-16-8-3-2-6-14(16)17-18(21(27)24-20(17)26)15-12-25(11-5-9-22)19-13(15)7-4-10-23-19/h2-4,6-8,10,12H,5,9,11,22H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147462

(3-(2-Chloro-phenyl)-4-[1-(3-dimethylamino-propyl)-...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2cccnc12 |t:9| Show InChI InChI=1S/C22H21ClN4O2/c1-26(2)11-6-12-27-13-16(14-8-5-10-24-20(14)27)19-18(21(28)25-22(19)29)15-7-3-4-9-17(15)23/h3-5,7-10,13H,6,11-12H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50147467

(3-[1-(3-Dimethylamino-propyl)-1H-pyrrolo[2,3-b]pyr...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2cccc3ccccc23)c2cccnc12 |t:9| Show InChI InChI=1S/C26H24N4O2/c1-29(2)14-7-15-30-16-21(20-12-6-13-27-24(20)30)23-22(25(31)28-26(23)32)19-11-5-9-17-8-3-4-10-18(17)19/h3-6,8-13,16H,7,14-15H2,1-2H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein kinase C-alpha using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147472

(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCO)c2ncccc12 |t:9| Show InChI InChI=1S/C21H19N3O4/c1-28-16-8-3-2-6-14(16)17-18(21(27)23-20(17)26)15-12-24(10-5-11-25)19-13(15)7-4-9-22-19/h2-4,6-9,12,25H,5,10-11H2,1H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50147458

((3-{3-[4-(2-Methoxy-phenyl)-2,5-dioxo-2,5-dihydro-...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC(=O)OC(C)(C)C)c2ncccc12 |t:9| Show InChI InChI=1S/C26H28N4O5/c1-26(2,3)35-25(33)28-13-8-14-30-15-18(16-10-7-12-27-22(16)30)21-20(23(31)29-24(21)32)17-9-5-6-11-19(17)34-4/h5-7,9-12,15H,8,13-14H2,1-4H3,(H,28,33)(H,29,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Protein tyrosine kinase Lyn |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147461

(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2C(F)(F)F)c2cccnc12 |t:7| Show InChI InChI=1S/C21H16F3N3O3/c22-21(23,24)15-7-2-1-5-13(15)16-17(20(30)26-19(16)29)14-11-27(9-4-10-28)18-12(14)6-3-8-25-18/h1-3,5-8,11,28H,4,9-10H2,(H,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147459

(CHEMBL326208 | N-(3-{3-[4-(2-Methoxy-phenyl)-2,5-d...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNS(C)(=O)=O)c2ncccc12 |t:9| Show InChI InChI=1S/C22H22N4O5S/c1-31-17-9-4-3-7-15(17)18-19(22(28)25-21(18)27)16-13-26(12-6-11-24-32(2,29)30)20-14(16)8-5-10-23-20/h3-5,7-10,13,24H,6,11-12H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50147457

(3-(2-Hydroxy-phenyl)-4-[1-(3-hydroxy-propyl)-1H-py...)Show SMILES COCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2O)c2cccnc12 |t:8| Show InChI InChI=1S/C21H19N3O4/c1-28-11-5-10-24-12-15(13-7-4-9-22-19(13)24)18-17(20(26)23-21(18)27)14-6-2-3-8-16(14)25/h2-4,6-9,12,25H,5,10-11H2,1H3,(H,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Ribosomal S6 kinase 3 |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147458

((3-{3-[4-(2-Methoxy-phenyl)-2,5-dioxo-2,5-dihydro-...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC(=O)OC(C)(C)C)c2ncccc12 |t:9| Show InChI InChI=1S/C26H28N4O5/c1-26(2,3)35-25(33)28-13-8-14-30-15-18(16-10-7-12-27-22(16)30)21-20(23(31)29-24(21)32)17-9-5-6-11-19(17)34-4/h5-7,9-12,15H,8,13-14H2,1-4H3,(H,28,33)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147465

(3-(2-Chloro-4-fluoro-phenyl)-4-[1-(3-hydroxy-propy...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccc(F)cc2Cl)c2cccnc12 |t:7| Show InChI InChI=1S/C20H15ClFN3O3/c21-15-9-11(22)4-5-13(15)16-17(20(28)24-19(16)27)14-10-25(7-2-8-26)18-12(14)3-1-6-23-18/h1,3-6,9-10,26H,2,7-8H2,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147464

(CHEMBL109977 | N-(3-{3-[4-(2-Methoxy-phenyl)-2,5-d...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC=O)c2ncccc12 |t:9| Show InChI InChI=1S/C22H20N4O4/c1-30-17-8-3-2-6-15(17)18-19(22(29)25-21(18)28)16-12-26(11-5-9-23-13-27)20-14(16)7-4-10-24-20/h2-4,6-8,10,12-13H,5,9,11H2,1H3,(H,23,27)(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50147463

(3-(3-Chloro-5-trifluoromethyl-pyridin-2-yl)-4-[1-(...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ncc(cc2Cl)C(F)(F)F)c2cccnc12 |t:7| Show InChI InChI=1S/C20H14ClF3N4O3/c21-13-7-10(20(22,23)24)8-26-16(13)15-14(18(30)27-19(15)31)12-9-28(5-2-6-29)17-11(12)3-1-4-25-17/h1,3-4,7-9,29H,2,5-6H2,(H,27,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 2-cyclin A |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50147467

(3-[1-(3-Dimethylamino-propyl)-1H-pyrrolo[2,3-b]pyr...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2cccc3ccccc23)c2cccnc12 |t:9| Show InChI InChI=1S/C26H24N4O2/c1-29(2)14-7-15-30-16-21(20-12-6-13-27-24(20)30)23-22(25(31)28-26(23)32)19-11-5-9-17-8-3-4-10-18(17)19/h3-6,8-13,16H,7,14-15H2,1-2H3,(H,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C-gamma |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50147462

(3-(2-Chloro-phenyl)-4-[1-(3-dimethylamino-propyl)-...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2cccnc12 |t:9| Show InChI InChI=1S/C22H21ClN4O2/c1-26(2)11-6-12-27-13-16(14-8-5-10-24-20(14)27)19-18(21(28)25-22(19)29)15-7-3-4-9-17(15)23/h3-5,7-10,13H,6,11-12H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C-gamma |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50147469

(3-[1-(3-Hydroxy-propyl)-1H-pyrrolo[2,3-b]pyridin-3...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2cccc3ccccc23)c2cccnc12 |t:7| Show InChI InChI=1S/C24H19N3O3/c28-13-5-12-27-14-19(18-10-4-11-25-22(18)27)21-20(23(29)26-24(21)30)17-9-3-7-15-6-1-2-8-16(15)17/h1-4,6-11,14,28H,5,12-13H2,(H,26,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein kinase C-alpha using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50147462

(3-(2-Chloro-phenyl)-4-[1-(3-dimethylamino-propyl)-...)Show SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2cccnc12 |t:9| Show InChI InChI=1S/C22H21ClN4O2/c1-26(2)11-6-12-27-13-16(14-8-5-10-24-20(14)27)19-18(21(28)25-22(19)29)15-7-3-4-9-17(15)23/h3-5,7-10,13H,6,11-12H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein kinase C-alpha using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50147457

(3-(2-Hydroxy-phenyl)-4-[1-(3-hydroxy-propyl)-1H-py...)Show SMILES COCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2O)c2cccnc12 |t:8| Show InChI InChI=1S/C21H19N3O4/c1-28-11-5-10-24-12-15(13-7-4-9-22-19(13)24)18-17(20(26)23-21(18)27)14-6-2-3-8-16(14)25/h2-4,6-9,12,25H,5,10-11H2,1H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human protein kinase C-betaII using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50147473

(3-(2-Chloro-phenyl)-4-[1-(3-hydroxy-propyl)-1H-pyr...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2cccnc12 |t:7| Show InChI InChI=1S/C20H16ClN3O3/c21-15-7-2-1-5-13(15)16-17(20(27)23-19(16)26)14-11-24(9-4-10-25)18-12(14)6-3-8-22-18/h1-3,5-8,11,25H,4,9-10H2,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein kinase C-alpha using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50147465

(3-(2-Chloro-4-fluoro-phenyl)-4-[1-(3-hydroxy-propy...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccc(F)cc2Cl)c2cccnc12 |t:7| Show InChI InChI=1S/C20H15ClFN3O3/c21-15-9-11(22)4-5-13(15)16-17(20(28)24-19(16)27)14-10-25(7-2-8-26)18-12(14)3-1-6-23-18/h1,3-6,9-10,26H,2,7-8H2,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein kinase C-alpha using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50147459

(CHEMBL326208 | N-(3-{3-[4-(2-Methoxy-phenyl)-2,5-d...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNS(C)(=O)=O)c2ncccc12 |t:9| Show InChI InChI=1S/C22H22N4O5S/c1-31-17-9-4-3-7-15(17)18-19(22(28)25-21(18)27)16-13-26(12-6-11-24-32(2,29)30)20-14(16)8-5-10-23-20/h3-5,7-10,13,24H,6,11-12H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein kinase C-alpha using histone as substrate |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50147473

(3-(2-Chloro-phenyl)-4-[1-(3-hydroxy-propyl)-1H-pyr...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2Cl)c2cccnc12 |t:7| Show InChI InChI=1S/C20H16ClN3O3/c21-15-7-2-1-5-13(15)16-17(20(27)23-19(16)26)14-11-24(9-4-10-25)18-12(14)6-3-8-22-18/h1-3,5-8,11,25H,4,9-10H2,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C-gamma |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3
(Homo sapiens (Human)) | BDBM50147463

(3-(3-Chloro-5-trifluoromethyl-pyridin-2-yl)-4-[1-(...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ncc(cc2Cl)C(F)(F)F)c2cccnc12 |t:7| Show InChI InChI=1S/C20H14ClF3N4O3/c21-13-7-10(20(22,23)24)8-26-16(13)15-14(18(30)27-19(15)31)12-9-28(5-2-6-29)17-11(12)3-1-4-25-17/h1,3-4,7-9,29H,2,5-6H2,(H,27,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin-dependent kinase 1-cyclin B |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50147468

(3-(7-Azaindolyl)-4-arylmaleimide analogue | CHEMBL...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNS(N)(=O)=O)c2ncccc12 |t:9| Show InChI InChI=1S/C21H21N5O5S/c1-31-16-8-3-2-6-14(16)17-18(21(28)25-20(17)27)15-12-26(11-5-10-24-32(22,29)30)19-13(15)7-4-9-23-19/h2-4,6-9,12,24H,5,10-11H2,1H3,(H2,22,29,30)(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C-gamma |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50147459

(CHEMBL326208 | N-(3-{3-[4-(2-Methoxy-phenyl)-2,5-d...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNS(C)(=O)=O)c2ncccc12 |t:9| Show InChI InChI=1S/C22H22N4O5S/c1-31-17-9-4-3-7-15(17)18-19(22(28)25-21(18)27)16-13-26(12-6-11-24-32(2,29)30)20-14(16)8-5-10-23-20/h3-5,7-10,13,24H,6,11-12H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C-gamma |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50147464

(CHEMBL109977 | N-(3-{3-[4-(2-Methoxy-phenyl)-2,5-d...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC=O)c2ncccc12 |t:9| Show InChI InChI=1S/C22H20N4O4/c1-30-17-8-3-2-6-15(17)18-19(22(29)25-21(18)28)16-12-26(11-5-9-23-13-27)20-14(16)7-4-10-24-20/h2-4,6-8,10,12-13H,5,9,11H2,1H3,(H,23,27)(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of protein kinase C-gamma |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50147463

(3-(3-Chloro-5-trifluoromethyl-pyridin-2-yl)-4-[1-(...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ncc(cc2Cl)C(F)(F)F)c2cccnc12 |t:7| Show InChI InChI=1S/C20H14ClF3N4O3/c21-13-7-10(20(22,23)24)8-26-16(13)15-14(18(30)27-19(15)31)12-9-28(5-2-6-29)17-11(12)3-1-4-25-17/h1,3-4,7-9,29H,2,5-6H2,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MSK-1 kinase |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8296
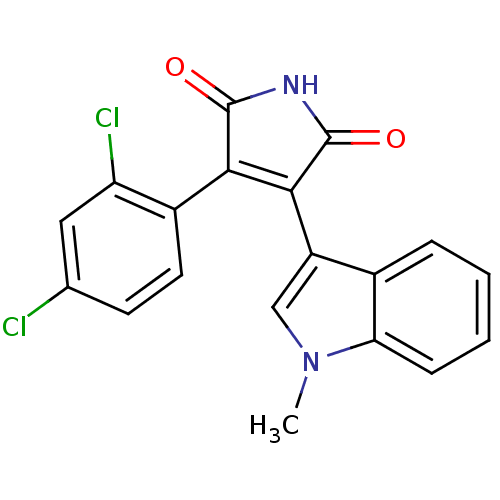
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Effective concentration of compound against glycogen synthase kinase-3 in HEK293 cells |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147463

(3-(3-Chloro-5-trifluoromethyl-pyridin-2-yl)-4-[1-(...)Show SMILES OCCCn1cc(C2=C(C(=O)NC2=O)c2ncc(cc2Cl)C(F)(F)F)c2cccnc12 |t:7| Show InChI InChI=1S/C20H14ClF3N4O3/c21-13-7-10(20(22,23)24)8-26-16(13)15-14(18(30)27-19(15)31)12-9-28(5-2-6-29)17-11(12)3-1-4-25-17/h1,3-4,7-9,29H,2,5-6H2,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 620 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Effective concentration of compound against glycogen synthase kinase-3 in HEK293 cells |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM26979
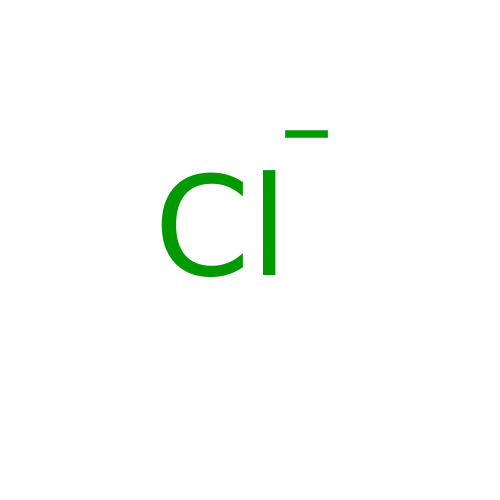
(CHEMBL69710 | Cl- | POTASSIUM CHLORIDE | SODIUM CH...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+6 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Effective concentration of compound against glycogen synthase kinase-3 in HEK293 cells |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147458

((3-{3-[4-(2-Methoxy-phenyl)-2,5-dioxo-2,5-dihydro-...)Show SMILES COc1ccccc1C1=C(C(=O)NC1=O)c1cn(CCCNC(=O)OC(C)(C)C)c2ncccc12 |t:9| Show InChI InChI=1S/C26H28N4O5/c1-26(2,3)35-25(33)28-13-8-14-30-15-18(16-10-7-12-27-22(16)30)21-20(23(31)29-24(21)32)17-9-5-6-11-19(17)34-4/h5-7,9-12,15H,8,13-14H2,1-4H3,(H,28,33)(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Effective concentration of compound against glycogen synthase kinase-3 in HEK293 cells |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50147457

(3-(2-Hydroxy-phenyl)-4-[1-(3-hydroxy-propyl)-1H-py...)Show SMILES COCCCn1cc(C2=C(C(=O)NC2=O)c2ccccc2O)c2cccnc12 |t:8| Show InChI InChI=1S/C21H19N3O4/c1-28-11-5-10-24-12-15(13-7-4-9-22-19(13)24)18-17(20(26)23-21(18)27)14-6-2-3-8-16(14)25/h2-4,6-9,12,25H,5,10-11H2,1H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
| Assay Description
Effective concentration of compound against glycogen synthase kinase-3 in HEK293 cells |
Bioorg Med Chem Lett 14: 3245-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.090
BindingDB Entry DOI: 10.7270/Q2W958NH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data