

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148073 (17-cyclopropylmethyl-3-methoxy-10-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148076 (17-cyclopropylmethyl-3-hydroxy-10-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148072 (4-cyclopropylmethyl-17-(3-phenylpropoxy)-(1S,5R,17...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148076 (17-cyclopropylmethyl-3-hydroxy-10-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148076 (17-cyclopropylmethyl-3-hydroxy-10-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148073 (17-cyclopropylmethyl-3-methoxy-10-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148071 ((-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148074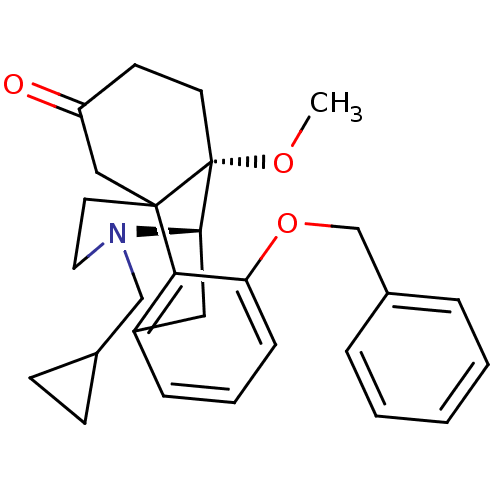 (3-benzyloxy-17-cyclopropylmethyl-10-methoxy-(1R,9R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148070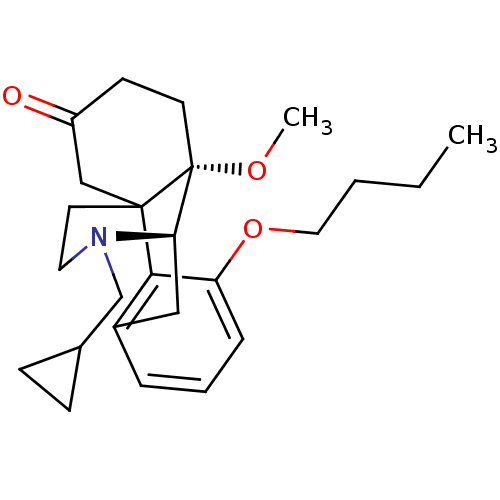 (3-butoxy-17-cyclopropylmethyl-10-methoxy-(1R,9R,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148079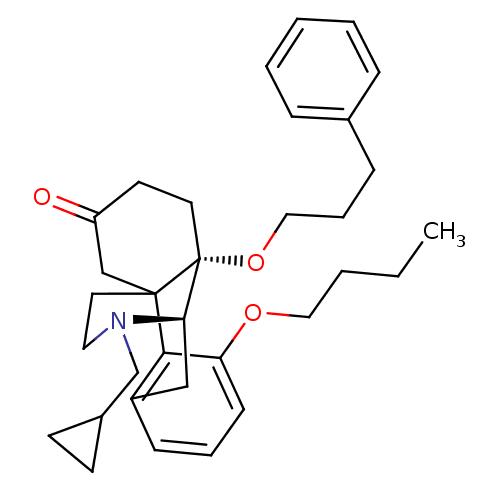 (3-butoxy-17-cyclopropylmethyl-10-(3-phenylpropoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148073 (17-cyclopropylmethyl-3-methoxy-10-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148072 (4-cyclopropylmethyl-17-(3-phenylpropoxy)-(1S,5R,17...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148078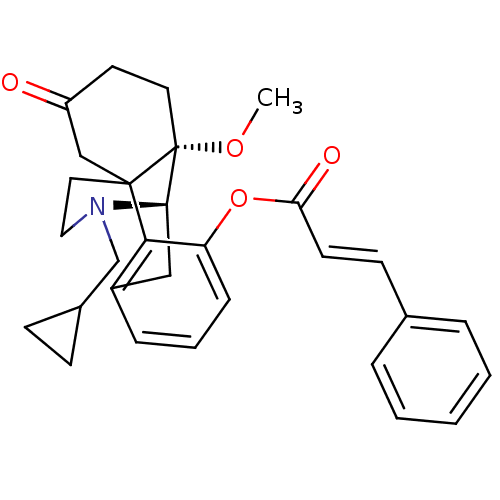 (17-cyclopropylmethyl-10-methoxy-13-oxo-(1R,9R,10S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148075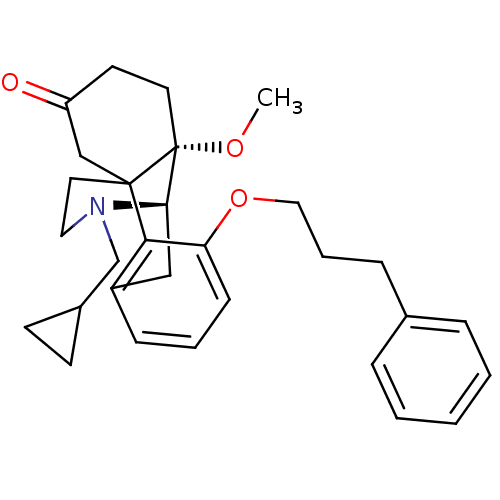 (17-cyclopropylmethyl-10-methoxy-3-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148077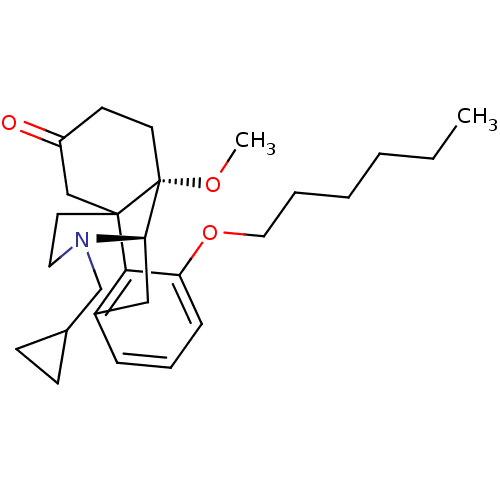 (17-cyclopropylmethyl-3-hexyloxy-10-methoxy-(1R,9R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]DAMGO from Opioid receptor mu 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148072 (4-cyclopropylmethyl-17-(3-phenylpropoxy)-(1S,5R,17...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148074 (3-benzyloxy-17-cyclopropylmethyl-10-methoxy-(1R,9R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148071 ((-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148079 (3-butoxy-17-cyclopropylmethyl-10-(3-phenylpropoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148075 (17-cyclopropylmethyl-10-methoxy-3-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148074 (3-benzyloxy-17-cyclopropylmethyl-10-methoxy-(1R,9R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148079 (3-butoxy-17-cyclopropylmethyl-10-(3-phenylpropoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148070 (3-butoxy-17-cyclopropylmethyl-10-methoxy-(1R,9R,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148078 (17-cyclopropylmethyl-10-methoxy-13-oxo-(1R,9R,10S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148071 ((-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148077 (17-cyclopropylmethyl-3-hexyloxy-10-methoxy-(1R,9R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H]U69,593 from Opioid receptor kappa 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148070 (3-butoxy-17-cyclopropylmethyl-10-methoxy-(1R,9R,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.65E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148078 (17-cyclopropylmethyl-10-methoxy-13-oxo-(1R,9R,10S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148077 (17-cyclopropylmethyl-3-hexyloxy-10-methoxy-(1R,9R,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148075 (17-cyclopropylmethyl-10-methoxy-3-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.41E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Binding affinity determined by displacing [3H][Ile5,6]deltorphin II from opioid receptor delta 1 in rat brain membranes | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50148070 (3-butoxy-17-cyclopropylmethyl-10-methoxy-(1R,9R,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor kappa 1 ex... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50148071 ((-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor mu 1 expre... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50148072 (4-cyclopropylmethyl-17-(3-phenylpropoxy)-(1S,5R,17...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor kappa 1 ex... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50148076 (17-cyclopropylmethyl-3-hydroxy-10-(3-phenylpropoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor mu 1 expre... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50148073 (17-cyclopropylmethyl-3-methoxy-10-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human opioid receptor delta 1 ex... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50148071 ((-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor kappa 1 ex... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50148076 (17-cyclopropylmethyl-3-hydroxy-10-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor kappa 1 ex... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50148071 ((-)-N-(Cycloproylmethyl)-4,14-dimethoxymorphinan-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human opioid receptor delta 1 ex... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50148073 (17-cyclopropylmethyl-3-methoxy-10-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor kappa 1 ex... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50148072 (4-cyclopropylmethyl-17-(3-phenylpropoxy)-(1S,5R,17...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human opioid receptor delta 1 ex... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50148072 (4-cyclopropylmethyl-17-(3-phenylpropoxy)-(1S,5R,17...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor mu 1 expre... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50148076 (17-cyclopropylmethyl-3-hydroxy-10-(3-phenylpropoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human opioid receptor delta 1 ex... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50148073 (17-cyclopropylmethyl-3-methoxy-10-(3-phenylpropoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor mu 1 expre... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50148070 (3-butoxy-17-cyclopropylmethyl-10-methoxy-(1R,9R,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human opioid receptor delta 1 ex... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50148070 (3-butoxy-17-cyclopropylmethyl-10-methoxy-(1R,9R,10...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Concentration necessary to produce 50% of the Emax value, i.e. to stimulate [35S]GTP-gamma-S, binding to recombinant human Opioid receptor mu 1 expre... | J Med Chem 47: 3242-7 (2004) Article DOI: 10.1021/jm031126k BindingDB Entry DOI: 10.7270/Q2TD9WT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||