

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13997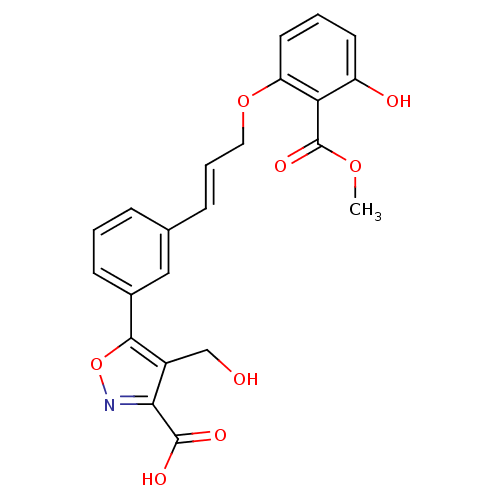 (5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl)phenoxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 920 | -34.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13996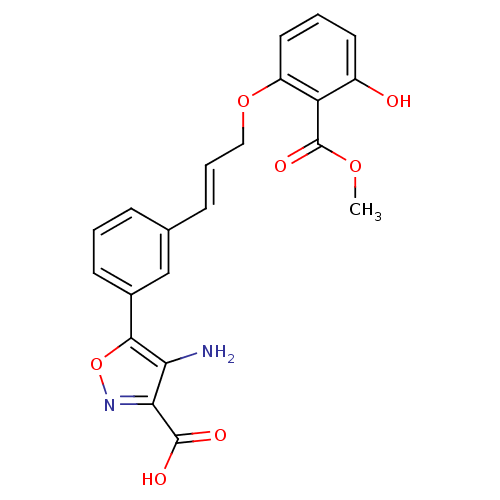 (4-amino-5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13990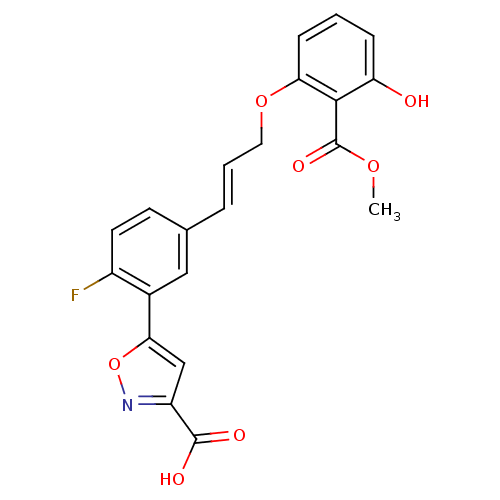 (5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 6.90E+3 | -29.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13995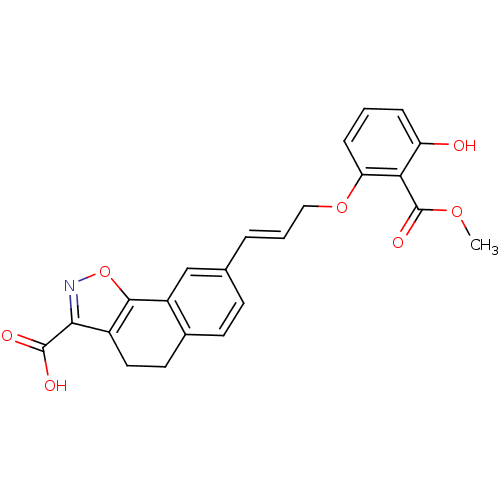 (12-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl)phenoxy]p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.15E+4 | -27.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13997 (5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl)phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.92E+4 | -26.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13994 (5-(3-{2-[3-hydroxy-2-(methoxycarbonyl)phenoxymethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13996 (4-amino-5-{3-[(1E)-3-[3-hydroxy-2-(methoxycarbonyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | >-25.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13993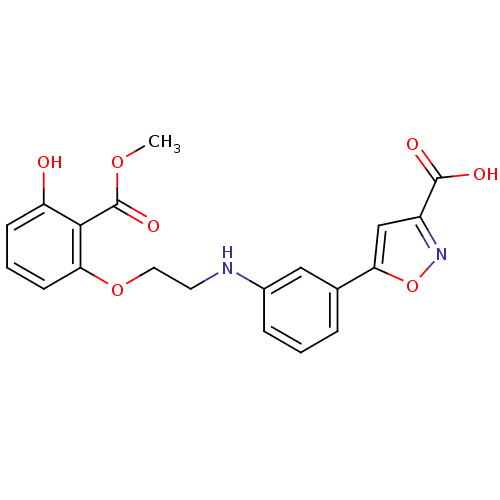 (5-[3-({2-[3-hydroxy-2-(methoxycarbonyl)phenoxy]eth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.03E+4 | -23.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13992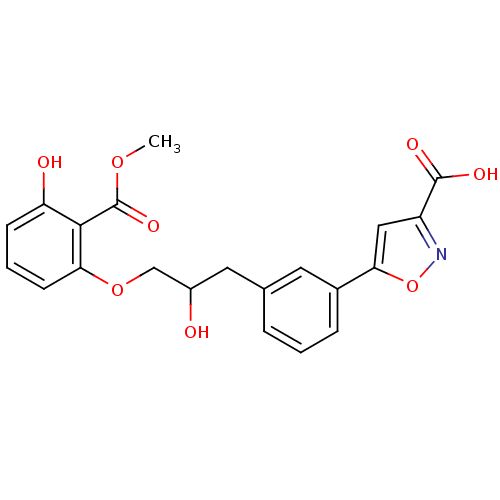 (5-(3-{2-hydroxy-3-[3-hydroxy-2-(methoxycarbonyl)ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.22E+5 | -22.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13990 (5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.64E+5 | -21.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13991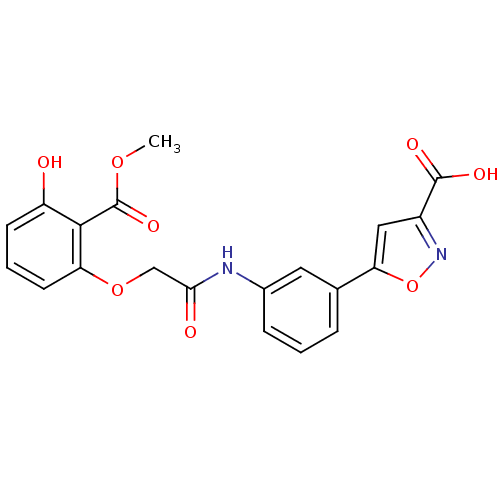 (5-(3-{2-[3-hydroxy-2-(methoxycarbonyl)phenoxy]acet...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.16E+5 | -20.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 14: 5543-6 (2004) Article DOI: 10.1016/j.bmcl.2004.08.063 BindingDB Entry DOI: 10.7270/Q2Z036D9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||