

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005459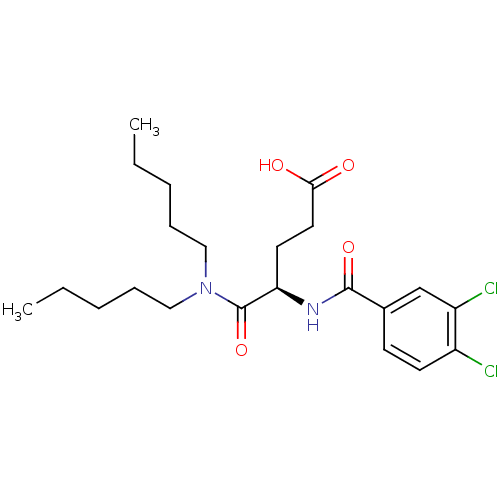 (4-(3,4-Dichloro-benzoylamino)-4-dipentylcarbamoyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005456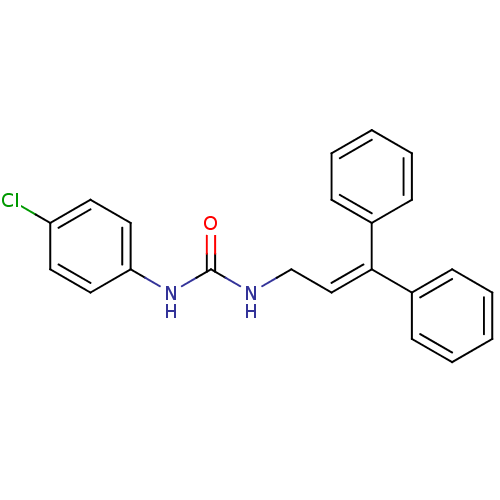 (1-(4-Chloro-phenyl)-3-(3,3-diphenyl-allyl)-urea | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005471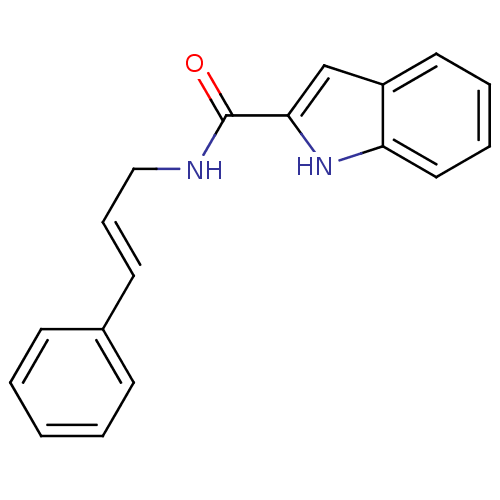 (1H-Indole-2-carboxylic acid (3-phenyl-allyl)-amide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005450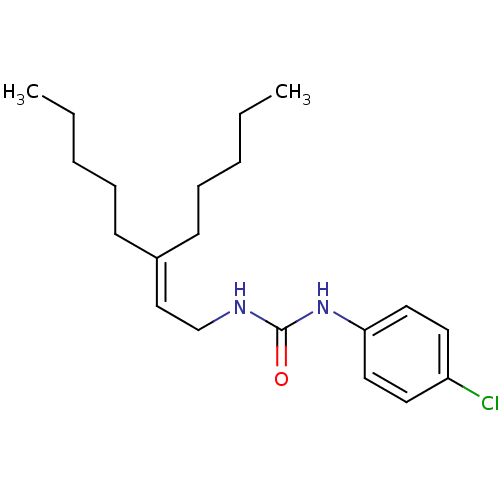 (1-(4-Chloro-phenyl)-3-(3-pentyl-oct-2-enyl)-urea |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005453 (1H-Indole-2-carboxylic acid [3,3-bis-(4-chloro-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005455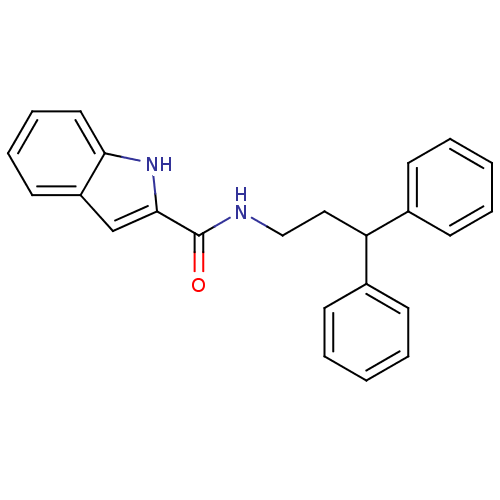 (1H-Indole-2-carboxylic acid (3,3-diphenyl-propyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005466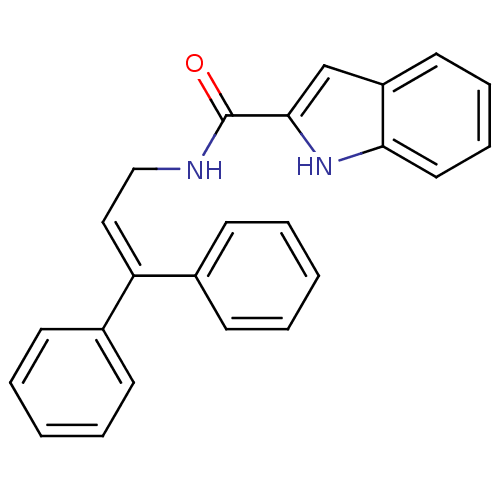 (1H-Indole-2-carboxylic acid (3,3-diphenyl-allyl)-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005468 (3,4-Dichloro-N-(3,3-diphenyl-allyl)-benzamide | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005457 (1-(4-Chloro-phenyl)-3-(4,4-diphenyl-but-3-enyl)-ur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005470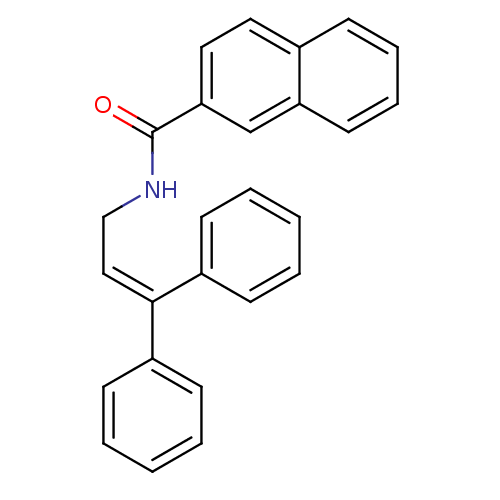 (CHEMBL8949 | Naphthalene-2-carboxylic acid (3,3-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005461 (CHEMBL9510 | Quinoline-2-carboxylic acid (3,3-diph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005469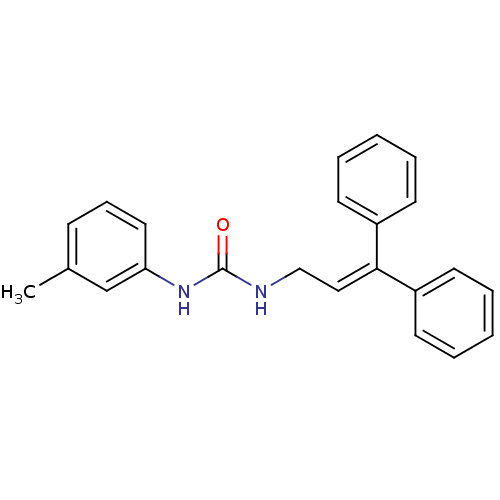 (1-(3,3-Diphenyl-allyl)-3-m-tolyl-urea | CHEMBL9312) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005462 (CHEMBL9379 | Naphthalene-1-carboxylic acid (3,3-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005467 (1-(4-Chloro-phenyl)-3-(2-diphenylamino-ethyl)-urea...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005449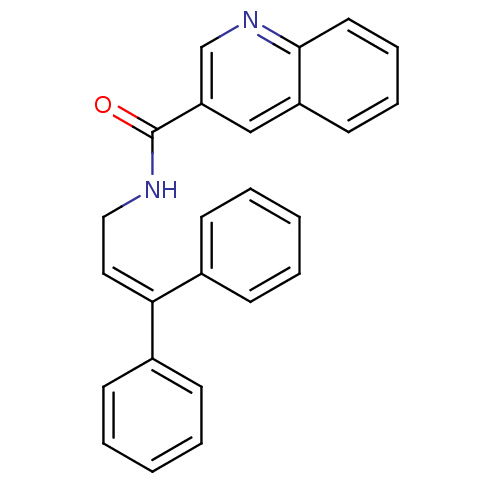 (CHEMBL9174 | Quinoline-3-carboxylic acid (3,3-diph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005454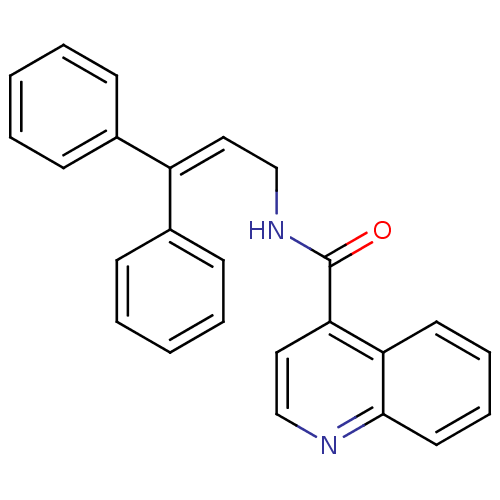 (CHEMBL9181 | Quinoline-4-carboxylic acid (3,3-diph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005452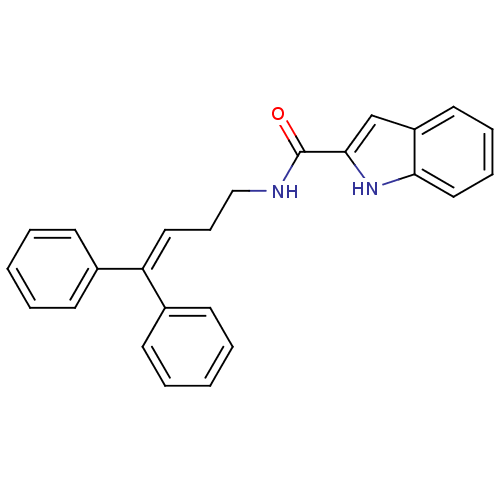 (1H-Indole-2-carboxylic acid (4,4-diphenyl-but-3-en...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit [3H]-L-364,718 binding Cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005465 (4-Chloro-N-(3,3-diphenyl-allyl)-benzamide | CHEMBL...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005460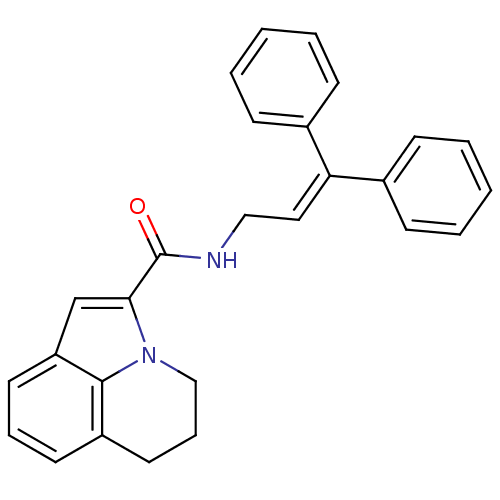 (5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinoline-2-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005458 (1-(4-Chloro-phenyl)-3-(3-phenyl-allyl)-urea | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005451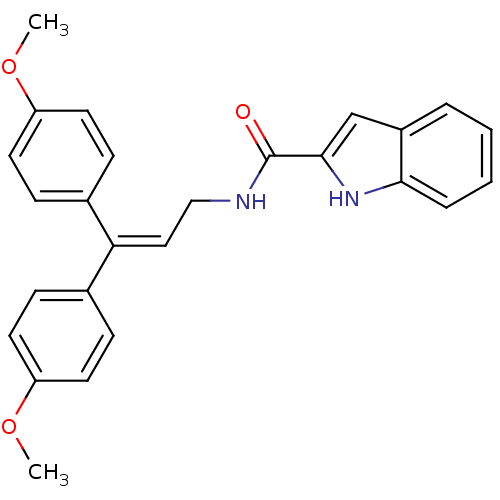 (1H-Indole-2-carboxylic acid [3,3-bis-(4-methoxy-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]-L-364,718 from Cholecystokinin type A receptor of rat pancreatic membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005464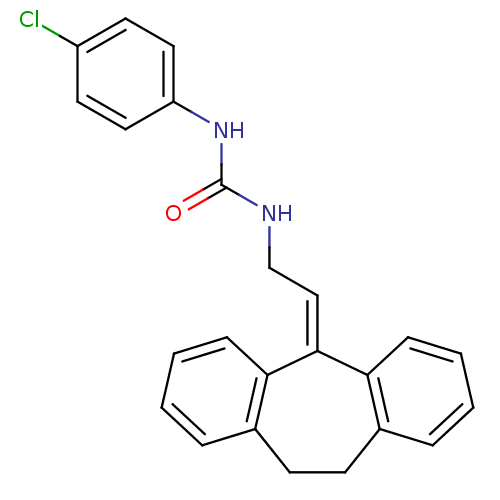 (1-(4-Chloro-phenyl)-3-[2-(10,11-dihydro-dibenzo[a,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of [3H]-L-364,718 binding to cholecystokinin type A receptor in rat pancreas membranes | J Med Chem 35: 1042-9 (1992) BindingDB Entry DOI: 10.7270/Q2HT2N8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||