 Found 46 hits of Enzyme Inhibition Constant Data
Found 46 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157055

((S)-3-{4-[3-(4-Benzoyl-phenoxy)-propoxy]-3-chloro-...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)C(=O)c2ccccc2)c(Cl)c1)C(O)=O Show InChI InChI=1S/C26H25ClO6/c1-31-24(26(29)30)17-18-8-13-23(22(27)16-18)33-15-5-14-32-21-11-9-20(10-12-21)25(28)19-6-3-2-4-7-19/h2-4,6-13,16,24H,5,14-15,17H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157059

((S)-3-{4-[3-(4-Benzoyl-phenoxy)-propoxy]-phenyl}-2...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)C(=O)c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H26O6/c1-30-24(26(28)29)18-19-8-12-22(13-9-19)31-16-5-17-32-23-14-10-21(11-15-23)25(27)20-6-3-2-4-7-20/h2-4,6-15,24H,5,16-18H2,1H3,(H,28,29)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157057

((S)-2-Methoxy-3-{4-[5-(4-phenoxy-phenoxy)-pent-1-y...)Show SMILES CO[C@@H](Cc1ccc(cc1)C#CCCCOc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C27H26O5/c1-30-26(27(28)29)20-22-13-11-21(12-14-22)8-4-3-7-19-31-23-15-17-25(18-16-23)32-24-9-5-2-6-10-24/h2,5-6,9-18,26H,3,7,19-20H2,1H3,(H,28,29)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157060

((S)-2-Methoxy-3-{4-[4-(4-phenoxy-phenoxy)-but-1-yn...)Show SMILES CO[C@@H](Cc1ccc(cc1)C#CCCOc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H24O5/c1-29-25(26(27)28)19-21-12-10-20(11-13-21)7-5-6-18-30-22-14-16-24(17-15-22)31-23-8-3-2-4-9-23/h2-4,8-17,25H,6,18-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157058
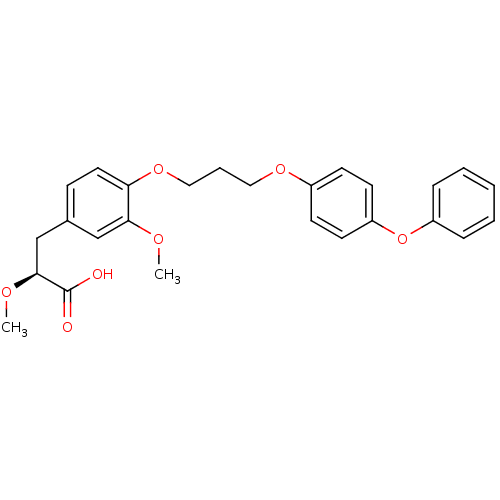
((S)-2-Methoxy-3-{3-methoxy-4-[3-(4-phenoxy-phenoxy...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)c(OC)c1)C(O)=O Show InChI InChI=1S/C26H28O7/c1-29-24-17-19(18-25(30-2)26(27)28)9-14-23(24)32-16-6-15-31-20-10-12-22(13-11-20)33-21-7-4-3-5-8-21/h3-5,7-14,17,25H,6,15-16,18H2,1-2H3,(H,27,28)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157061

((S)-3-{4-[3-(Biphenyl-4-yloxy)-propoxy]-phenyl}-2-...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C25H26O5/c1-28-24(25(26)27)18-19-8-12-22(13-9-19)29-16-5-17-30-23-14-10-21(11-15-23)20-6-3-2-4-7-20/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157056

((S)-2-Methoxy-3-{4-[3-(4-phenoxy-phenoxy)-propoxy]...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C25H26O6/c1-28-24(25(26)27)18-19-8-10-20(11-9-19)29-16-5-17-30-21-12-14-23(15-13-21)31-22-6-3-2-4-7-22/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157054

((S)-2-Ethoxy-3-{4-[3-(4-phenoxy-phenoxy)-propoxy]-...)Show SMILES CCO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C26H28O6/c1-2-29-25(26(27)28)19-20-9-11-21(12-10-20)30-17-6-18-31-22-13-15-24(16-14-22)32-23-7-4-3-5-8-23/h3-5,7-16,25H,2,6,17-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157055

((S)-3-{4-[3-(4-Benzoyl-phenoxy)-propoxy]-3-chloro-...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)C(=O)c2ccccc2)c(Cl)c1)C(O)=O Show InChI InChI=1S/C26H25ClO6/c1-31-24(26(29)30)17-18-8-13-23(22(27)16-18)33-15-5-14-32-21-11-9-20(10-12-21)25(28)19-6-3-2-4-7-19/h2-4,6-13,16,24H,5,14-15,17H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 309 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157059

((S)-3-{4-[3-(4-Benzoyl-phenoxy)-propoxy]-phenyl}-2...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)C(=O)c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H26O6/c1-30-24(26(28)29)18-19-8-12-22(13-9-19)31-16-5-17-32-23-14-10-21(11-15-23)25(27)20-6-3-2-4-7-20/h2-4,6-15,24H,5,16-18H2,1H3,(H,28,29)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157061

((S)-3-{4-[3-(Biphenyl-4-yloxy)-propoxy]-phenyl}-2-...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C25H26O5/c1-28-24(25(26)27)18-19-8-12-22(13-9-19)29-16-5-17-30-23-14-10-21(11-15-23)20-6-3-2-4-7-20/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 568 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157057

((S)-2-Methoxy-3-{4-[5-(4-phenoxy-phenoxy)-pent-1-y...)Show SMILES CO[C@@H](Cc1ccc(cc1)C#CCCCOc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C27H26O5/c1-30-26(27(28)29)20-22-13-11-21(12-14-22)8-4-3-7-19-31-23-15-17-25(18-16-23)32-24-9-5-2-6-10-24/h2,5-6,9-18,26H,3,7,19-20H2,1H3,(H,28,29)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157060

((S)-2-Methoxy-3-{4-[4-(4-phenoxy-phenoxy)-but-1-yn...)Show SMILES CO[C@@H](Cc1ccc(cc1)C#CCCOc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H24O5/c1-29-25(26(27)28)19-21-12-10-20(11-13-21)7-5-6-18-30-22-14-16-24(17-15-22)31-23-8-3-2-4-9-23/h2-4,8-17,25H,6,18-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157058

((S)-2-Methoxy-3-{3-methoxy-4-[3-(4-phenoxy-phenoxy...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)c(OC)c1)C(O)=O Show InChI InChI=1S/C26H28O7/c1-29-24-17-19(18-25(30-2)26(27)28)9-14-23(24)32-16-6-15-31-20-10-12-22(13-11-20)33-21-7-4-3-5-8-21/h3-5,7-14,17,25H,6,15-16,18H2,1-2H3,(H,27,28)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50157061

((S)-3-{4-[3-(Biphenyl-4-yloxy)-propoxy]-phenyl}-2-...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C25H26O5/c1-28-24(25(26)27)18-19-8-12-22(13-9-19)29-16-5-17-30-23-14-10-21(11-15-23)20-6-3-2-4-7-20/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor delta |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50157055

((S)-3-{4-[3-(4-Benzoyl-phenoxy)-propoxy]-3-chloro-...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)C(=O)c2ccccc2)c(Cl)c1)C(O)=O Show InChI InChI=1S/C26H25ClO6/c1-31-24(26(29)30)17-18-8-13-23(22(27)16-18)33-15-5-14-32-21-11-9-20(10-12-21)25(28)19-6-3-2-4-7-19/h2-4,6-13,16,24H,5,14-15,17H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor delta |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50157054

((S)-2-Ethoxy-3-{4-[3-(4-phenoxy-phenoxy)-propoxy]-...)Show SMILES CCO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C26H28O6/c1-2-29-25(26(27)28)19-20-9-11-21(12-10-20)30-17-6-18-31-22-13-15-24(16-14-22)32-23-7-4-3-5-8-23/h3-5,7-16,25H,2,6,17-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor delta |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157054

((S)-2-Ethoxy-3-{4-[3-(4-phenoxy-phenoxy)-propoxy]-...)Show SMILES CCO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C26H28O6/c1-2-29-25(26(27)28)19-20-9-11-21(12-10-20)30-17-6-18-31-22-13-15-24(16-14-22)32-23-7-4-3-5-8-23/h3-5,7-16,25H,2,6,17-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50157056

((S)-2-Methoxy-3-{4-[3-(4-phenoxy-phenoxy)-propoxy]...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C25H26O6/c1-28-24(25(26)27)18-19-8-10-20(11-9-19)29-16-5-17-30-21-12-14-23(15-13-21)31-22-6-3-2-4-7-22/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor delta |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50157059

((S)-3-{4-[3-(4-Benzoyl-phenoxy)-propoxy]-phenyl}-2...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)C(=O)c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H26O6/c1-30-24(26(28)29)18-19-8-12-22(13-9-19)31-16-5-17-32-23-14-10-21(11-15-23)25(27)20-6-3-2-4-7-20/h2-4,6-15,24H,5,16-18H2,1H3,(H,28,29)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor delta |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157056

((S)-2-Methoxy-3-{4-[3-(4-phenoxy-phenoxy)-propoxy]...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C25H26O6/c1-28-24(25(26)27)18-19-8-10-20(11-9-19)29-16-5-17-30-21-12-14-23(15-13-21)31-22-6-3-2-4-7-22/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50157060

((S)-2-Methoxy-3-{4-[4-(4-phenoxy-phenoxy)-but-1-yn...)Show SMILES CO[C@@H](Cc1ccc(cc1)C#CCCOc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H24O5/c1-29-25(26(27)28)19-21-12-10-20(11-13-21)7-5-6-18-30-22-14-16-24(17-15-22)31-23-8-3-2-4-9-23/h2-4,8-17,25H,6,18-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor delta |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50157057

((S)-2-Methoxy-3-{4-[5-(4-phenoxy-phenoxy)-pent-1-y...)Show SMILES CO[C@@H](Cc1ccc(cc1)C#CCCCOc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C27H26O5/c1-30-26(27(28)29)20-22-13-11-21(12-14-22)8-4-3-7-19-31-23-15-17-25(18-16-23)32-24-9-5-2-6-10-24/h2,5-6,9-18,26H,3,7,19-20H2,1H3,(H,28,29)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor delta |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50157058

((S)-2-Methoxy-3-{3-methoxy-4-[3-(4-phenoxy-phenoxy...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)c(OC)c1)C(O)=O Show InChI InChI=1S/C26H28O7/c1-29-24-17-19(18-25(30-2)26(27)28)9-14-23(24)32-16-6-15-31-20-10-12-22(13-11-20)33-21-7-4-3-5-8-21/h3-5,7-14,17,25H,6,15-16,18H2,1-2H3,(H,27,28)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor delta |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM28700
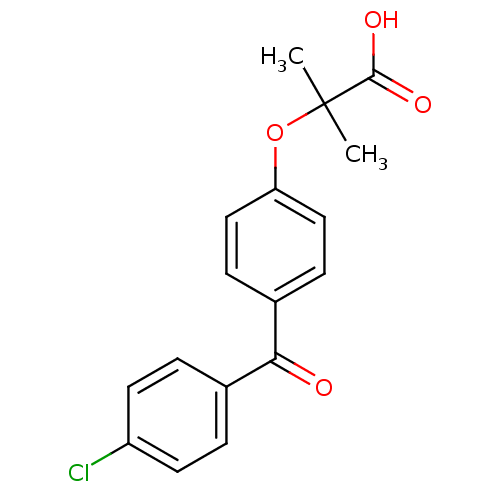
(2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropionic a...)Show InChI InChI=1S/C17H15ClO4/c1-17(2,16(20)21)22-14-9-5-12(6-10-14)15(19)11-3-7-13(18)8-4-11/h3-10H,1-2H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28700

(2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropionic a...)Show InChI InChI=1S/C17H15ClO4/c1-17(2,16(20)21)22-14-9-5-12(6-10-14)15(19)11-3-7-13(18)8-4-11/h3-10H,1-2H3,(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean inhibitory concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157054

((S)-2-Ethoxy-3-{4-[3-(4-phenoxy-phenoxy)-propoxy]-...)Show SMILES CCO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C26H28O6/c1-2-29-25(26(27)28)19-20-9-11-21(12-10-20)30-17-6-18-31-22-13-15-24(16-14-22)32-23-7-4-3-5-8-23/h3-5,7-16,25H,2,6,17-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157054

((S)-2-Ethoxy-3-{4-[3-(4-phenoxy-phenoxy)-propoxy]-...)Show SMILES CCO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C26H28O6/c1-2-29-25(26(27)28)19-20-9-11-21(12-10-20)30-17-6-18-31-22-13-15-24(16-14-22)32-23-7-4-3-5-8-23/h3-5,7-16,25H,2,6,17-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 282 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157060

((S)-2-Methoxy-3-{4-[4-(4-phenoxy-phenoxy)-but-1-yn...)Show SMILES CO[C@@H](Cc1ccc(cc1)C#CCCOc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H24O5/c1-29-25(26(27)28)19-21-12-10-20(11-13-21)7-5-6-18-30-22-14-16-24(17-15-22)31-23-8-3-2-4-9-23/h2-4,8-17,25H,6,18-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157058

((S)-2-Methoxy-3-{3-methoxy-4-[3-(4-phenoxy-phenoxy...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)c(OC)c1)C(O)=O Show InChI InChI=1S/C26H28O7/c1-29-24-17-19(18-25(30-2)26(27)28)9-14-23(24)32-16-6-15-31-20-10-12-22(13-11-20)33-21-7-4-3-5-8-21/h3-5,7-14,17,25H,6,15-16,18H2,1-2H3,(H,27,28)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157058

((S)-2-Methoxy-3-{3-methoxy-4-[3-(4-phenoxy-phenoxy...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)c(OC)c1)C(O)=O Show InChI InChI=1S/C26H28O7/c1-29-24-17-19(18-25(30-2)26(27)28)9-14-23(24)32-16-6-15-31-20-10-12-22(13-11-20)33-21-7-4-3-5-8-21/h3-5,7-14,17,25H,6,15-16,18H2,1-2H3,(H,27,28)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 185 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157061

((S)-3-{4-[3-(Biphenyl-4-yloxy)-propoxy]-phenyl}-2-...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C25H26O5/c1-28-24(25(26)27)18-19-8-12-22(13-9-19)29-16-5-17-30-23-14-10-21(11-15-23)20-6-3-2-4-7-20/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157056

((S)-2-Methoxy-3-{4-[3-(4-phenoxy-phenoxy)-propoxy]...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C25H26O6/c1-28-24(25(26)27)18-19-8-10-20(11-9-19)29-16-5-17-30-21-12-14-23(15-13-21)31-22-6-3-2-4-7-22/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 268 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM28700

(2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropionic a...)Show InChI InChI=1S/C17H15ClO4/c1-17(2,16(20)21)22-14-9-5-12(6-10-14)15(19)11-3-7-13(18)8-4-11/h3-10H,1-2H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157056

((S)-2-Methoxy-3-{4-[3-(4-phenoxy-phenoxy)-propoxy]...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(Oc3ccccc3)cc2)cc1)C(O)=O Show InChI InChI=1S/C25H26O6/c1-28-24(25(26)27)18-19-8-10-20(11-9-19)29-16-5-17-30-21-12-14-23(15-13-21)31-22-6-3-2-4-7-22/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 361 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157061

((S)-3-{4-[3-(Biphenyl-4-yloxy)-propoxy]-phenyl}-2-...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)-c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C25H26O5/c1-28-24(25(26)27)18-19-8-12-22(13-9-19)29-16-5-17-30-23-14-10-21(11-15-23)20-6-3-2-4-7-20/h2-4,6-15,24H,5,16-18H2,1H3,(H,26,27)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 154 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157059

((S)-3-{4-[3-(4-Benzoyl-phenoxy)-propoxy]-phenyl}-2...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)C(=O)c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H26O6/c1-30-24(26(28)29)18-19-8-12-22(13-9-19)31-16-5-17-32-23-14-10-21(11-15-23)25(27)20-6-3-2-4-7-20/h2-4,6-15,24H,5,16-18H2,1H3,(H,28,29)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157059

((S)-3-{4-[3-(4-Benzoyl-phenoxy)-propoxy]-phenyl}-2...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)C(=O)c2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H26O6/c1-30-24(26(28)29)18-19-8-12-22(13-9-19)31-16-5-17-32-23-14-10-21(11-15-23)25(27)20-6-3-2-4-7-20/h2-4,6-15,24H,5,16-18H2,1H3,(H,28,29)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.87E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157057

((S)-2-Methoxy-3-{4-[5-(4-phenoxy-phenoxy)-pent-1-y...)Show SMILES CO[C@@H](Cc1ccc(cc1)C#CCCCOc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C27H26O5/c1-30-26(27(28)29)20-22-13-11-21(12-14-22)8-4-3-7-19-31-23-15-17-25(18-16-23)32-24-9-5-2-6-10-24/h2,5-6,9-18,26H,3,7,19-20H2,1H3,(H,28,29)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 239 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157055

((S)-3-{4-[3-(4-Benzoyl-phenoxy)-propoxy]-3-chloro-...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)C(=O)c2ccccc2)c(Cl)c1)C(O)=O Show InChI InChI=1S/C26H25ClO6/c1-31-24(26(29)30)17-18-8-13-23(22(27)16-18)33-15-5-14-32-21-11-9-20(10-12-21)25(28)19-6-3-2-4-7-19/h2-4,6-13,16,24H,5,14-15,17H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 305 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157060

((S)-2-Methoxy-3-{4-[4-(4-phenoxy-phenoxy)-but-1-yn...)Show SMILES CO[C@@H](Cc1ccc(cc1)C#CCCOc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C26H24O5/c1-29-25(26(27)28)19-21-12-10-20(11-13-21)7-5-6-18-30-22-14-16-24(17-15-22)31-23-8-3-2-4-9-23/h2-4,8-17,25H,6,18-19H2,1H3,(H,27,28)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 103 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator-activated receptor gamma |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157057

((S)-2-Methoxy-3-{4-[5-(4-phenoxy-phenoxy)-pent-1-y...)Show SMILES CO[C@@H](Cc1ccc(cc1)C#CCCCOc1ccc(Oc2ccccc2)cc1)C(O)=O Show InChI InChI=1S/C27H26O5/c1-30-26(27(28)29)20-22-13-11-21(12-14-22)8-4-3-7-19-31-23-15-17-25(18-16-23)32-24-9-5-2-6-10-24/h2,5-6,9-18,26H,3,7,19-20H2,1H3,(H,28,29)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.62E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50157055

((S)-3-{4-[3-(4-Benzoyl-phenoxy)-propoxy]-3-chloro-...)Show SMILES CO[C@@H](Cc1ccc(OCCCOc2ccc(cc2)C(=O)c2ccccc2)c(Cl)c1)C(O)=O Show InChI InChI=1S/C26H25ClO6/c1-31-24(26(29)30)17-18-8-13-23(22(27)16-18)33-15-5-14-32-21-11-9-20(10-12-21)25(28)19-6-3-2-4-7-19/h2-4,6-13,16,24H,5,14-15,17H2,1H3,(H,29,30)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Mean effective concentration against human peroxisome proliferator activated receptor alpha |
Bioorg Med Chem Lett 15: 51-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.042
BindingDB Entry DOI: 10.7270/Q24B30T6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data