

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005688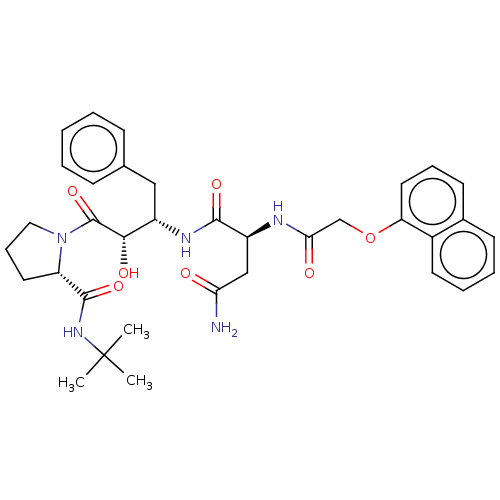 (CHEMBL3085518 | N*1*-[1-Benzyl-3-(2-tert-butylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Binding affinity against HIV Protease enzyme.(by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005701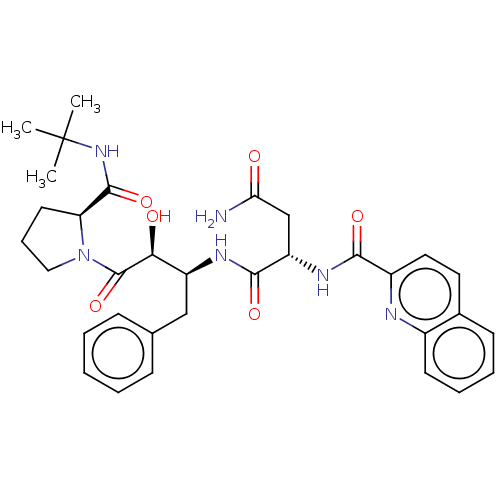 (CHEMBL337283 | N*1*-[1-Benzyl-3-(2-tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Binding affinity against HIV protease enzyme.(by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4215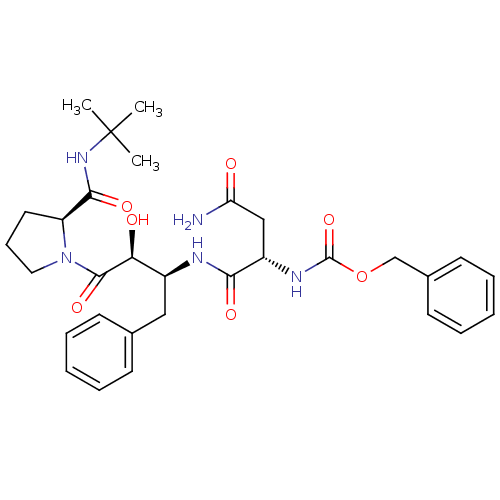 (AHPBA 1a | benzyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-2-(t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Binding affinity against HIV Protease enzyme.(by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005688 (CHEMBL3085518 | N*1*-[1-Benzyl-3-(2-tert-butylcarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005701 (CHEMBL337283 | N*1*-[1-Benzyl-3-(2-tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme (by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005694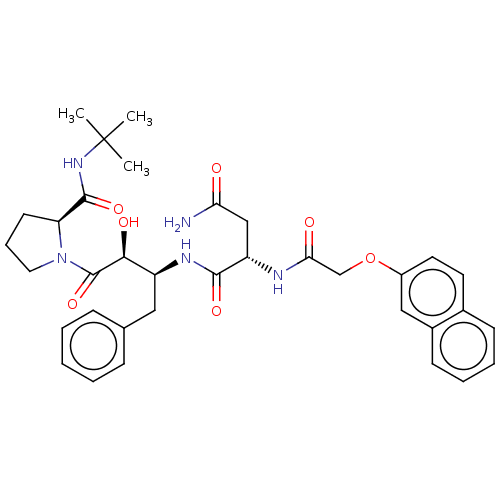 (CHEMBL334985 | N*1*-[1-Benzyl-3-(2-tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005695 (1-(2-Hydroxy-3-{3-methyl-2-[2-(naphthalen-1-yloxy)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50230656 (CHEMBL3349989) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368601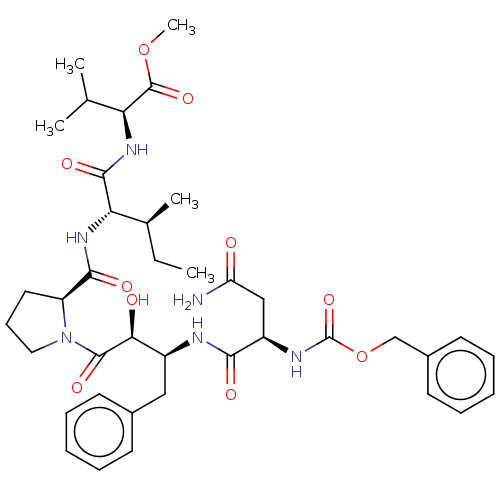 (CHEMBL1794048 | CHEMBL3349509) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50230655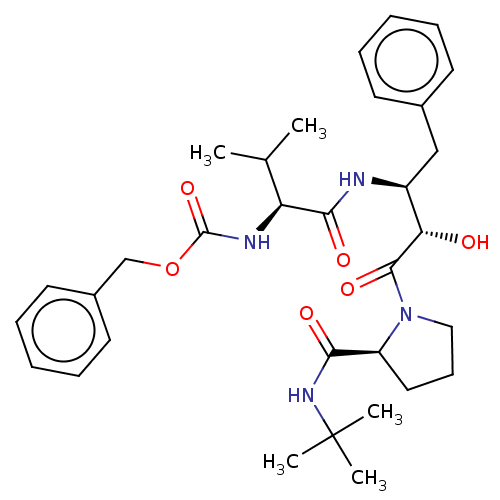 (CHEMBL3349662) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme.(value reported by Rich etal) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4215 (AHPBA 1a | benzyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-2-(t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005698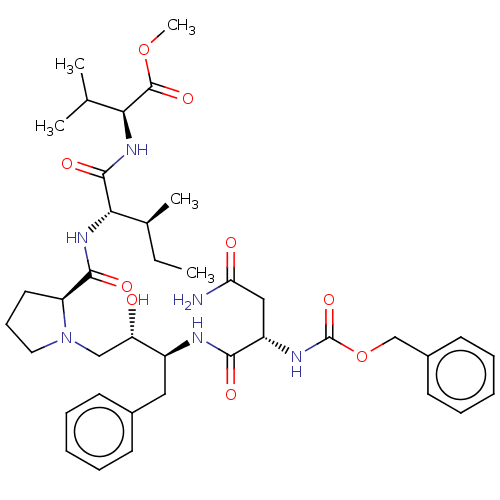 (2-[2-({1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme.(value reported by Roche group) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50011688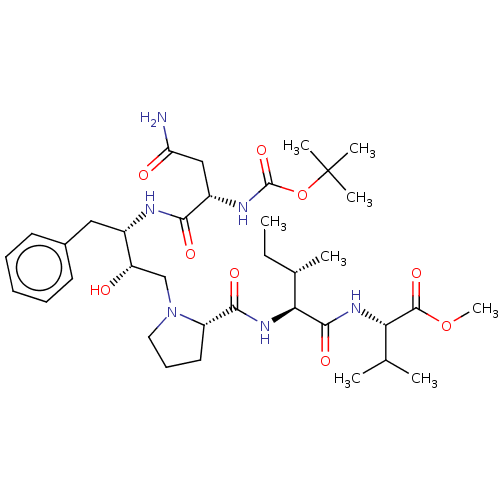 (Boc-Asn-Phe-HEA(R)-Pro-Ile-Val-OMe | CHEMBL3349554) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50283721 (CHEMBL93082 | {(S)-1-[(1S,2R)-1-Benzyl-3-((S)-2-te...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme.(value reported by Roche group) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005689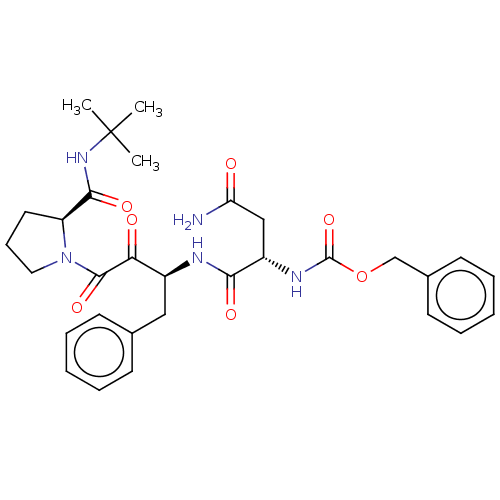 (BDBM50456284 | CHEMBL3085515 | {1-[1-Benzyl-3-(2-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005696 (2-[2-({1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005700 (CHEMBL3085514 | benzyl N-[(1S)-1-{[(2S)-4-[2-(tert...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407048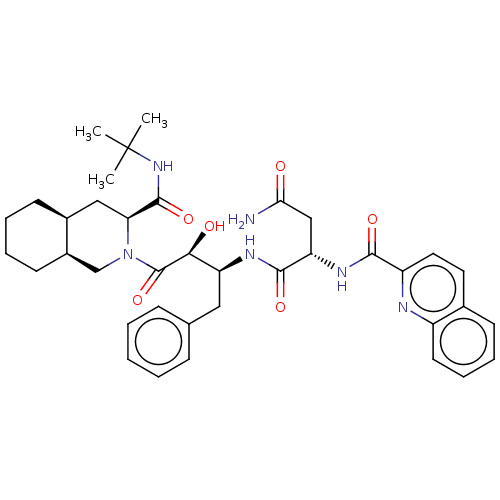 (CHEMBL3085513) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005687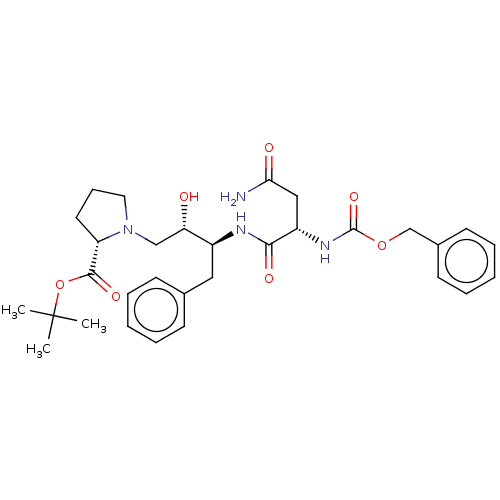 (1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme (by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407034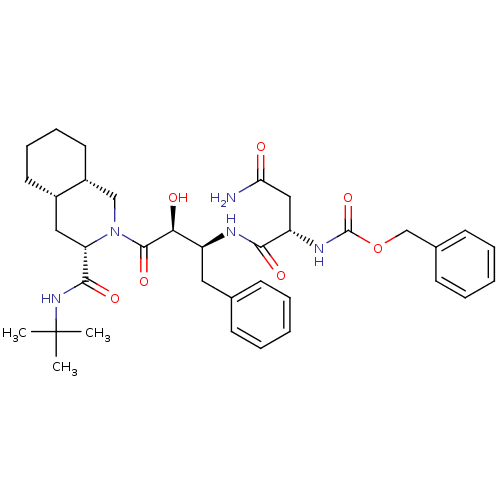 (CHEMBL132945) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005703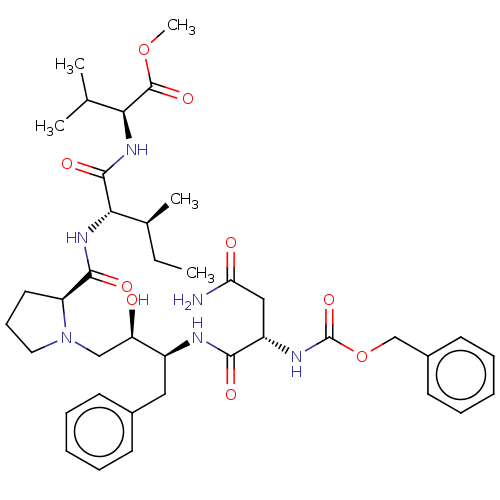 (2-[2-({1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme.(value reported by Roche group) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005687 (1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005687 (1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005687 (1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Binding affinity against HIV Protease enzyme.(by Dixon analysis) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005693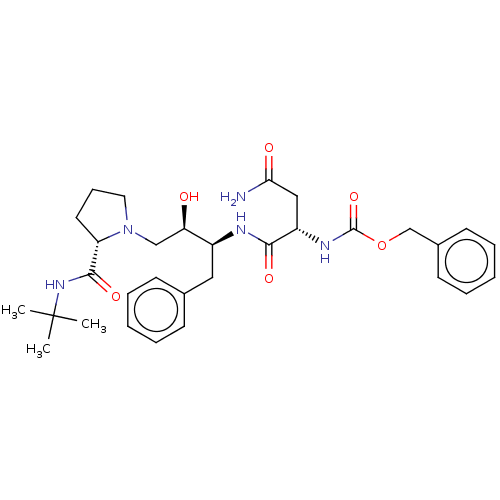 (CHEMBL3349988 | {1-[1-Benzyl-3-(2-tert-butylcarbam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme.(value reported by Roche group) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005687 (1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme.(value reported by Roche group) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005687 (1-[3-(2-Benzyloxycarbonylamino-3-carbamoyl-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme.(value reported by Roche group) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005702 (BDBM50407040 | CHEMBL336818 | [1-Benzyl-3-(2-tert-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005704 (BDBM50407027 | CHEMBL335023 | [1-Benzyl-3-(2-tert-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50011683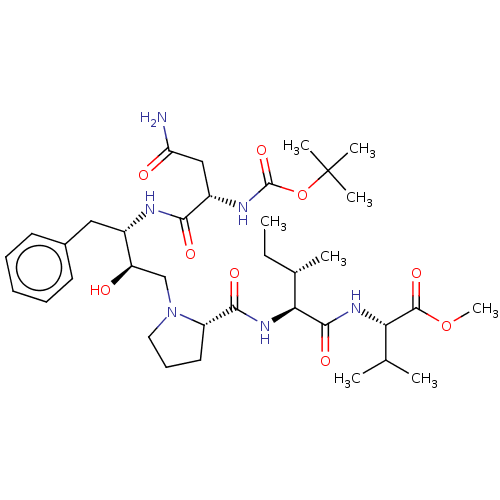 (Boc-Asn-Phe-HEA(S)-Pro-Ile-Val-OMe | CHEMBL3349553) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50407062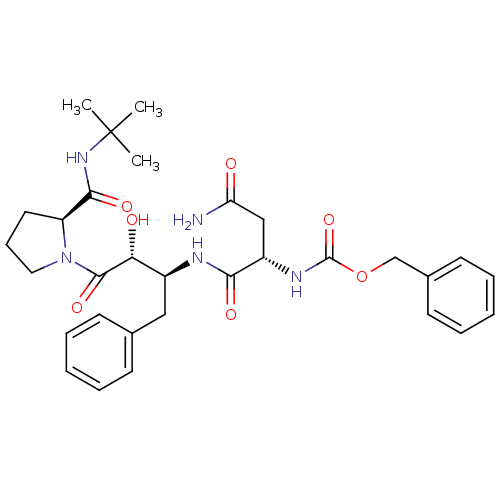 (CHEMBL335676) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme. | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50005705 (1-(3-Benzyloxycarbonylamino-2-hydroxy-4-phenyl-but...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Canada Curated by ChEMBL | Assay Description Inhibitory activity of the Compound was tested against HIV protease enzyme.(value reported by Roche group) | J Med Chem 35: 1318-20 (1992) BindingDB Entry DOI: 10.7270/Q2W959T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||