 Found 29 hits of Enzyme Inhibition Constant Data
Found 29 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146832
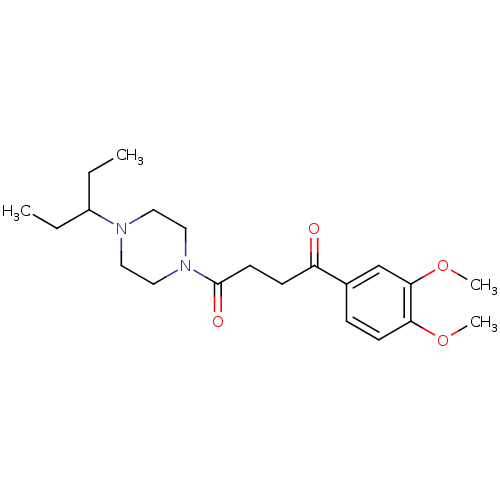
(1-(3,4-Dimethoxy-phenyl)-4-[4-(1-ethyl-propyl)-pip...)Show SMILES CCC(CC)N1CCN(CC1)C(=O)CCC(=O)c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C21H32N2O4/c1-5-17(6-2)22-11-13-23(14-12-22)21(25)10-8-18(24)16-7-9-19(26-3)20(15-16)27-4/h7,9,15,17H,5-6,8,10-14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159113
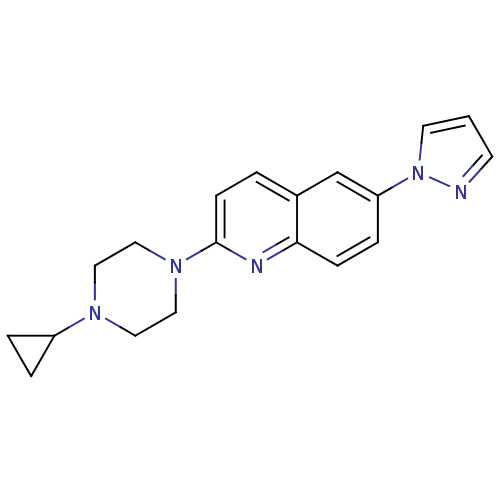
(2-(4-Cyclopropyl-piperazin-1-yl)-6-pyrazol-1-yl-qu...)Show InChI InChI=1S/C19H21N5/c1-8-20-24(9-1)17-5-6-18-15(14-17)2-7-19(21-18)23-12-10-22(11-13-23)16-3-4-16/h1-2,5-9,14,16H,3-4,10-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159114

(CHEMBL179234 | Cyclopropyl-[2-(4-cyclopropyl-piper...)Show InChI InChI=1S/C20H23N3O/c24-20(14-1-2-14)16-3-7-18-15(13-16)4-8-19(21-18)23-11-9-22(10-12-23)17-5-6-17/h3-4,7-8,13-14,17H,1-2,5-6,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50120543
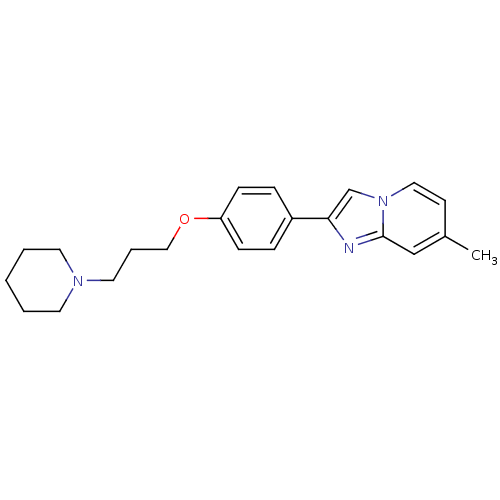
(7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...)Show InChI InChI=1S/C22H27N3O/c1-18-10-14-25-17-21(23-22(25)16-18)19-6-8-20(9-7-19)26-15-5-13-24-11-3-2-4-12-24/h6-10,14,16-17H,2-5,11-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159116
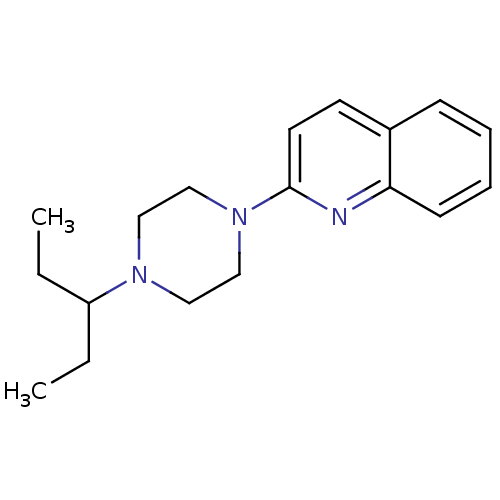
(2-[4-(1-Ethyl-propyl)-piperazin-1-yl]-quinoline | ...)Show InChI InChI=1S/C18H25N3/c1-3-16(4-2)20-11-13-21(14-12-20)18-10-9-15-7-5-6-8-17(15)19-18/h5-10,16H,3-4,11-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159122
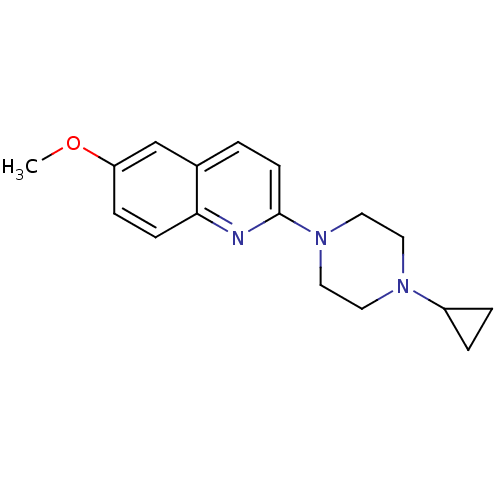
(2-(4-Cyclopropyl-piperazin-1-yl)-6-methoxy-quinoli...)Show InChI InChI=1S/C17H21N3O/c1-21-15-5-6-16-13(12-15)2-7-17(18-16)20-10-8-19(9-11-20)14-3-4-14/h2,5-7,12,14H,3-4,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159121

(2-(4-Cyclopropyl-piperazin-1-yl)-quinoline-6-carbo...)Show InChI InChI=1S/C17H18N4/c18-12-13-1-5-16-14(11-13)2-6-17(19-16)21-9-7-20(8-10-21)15-3-4-15/h1-2,5-6,11,15H,3-4,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159120
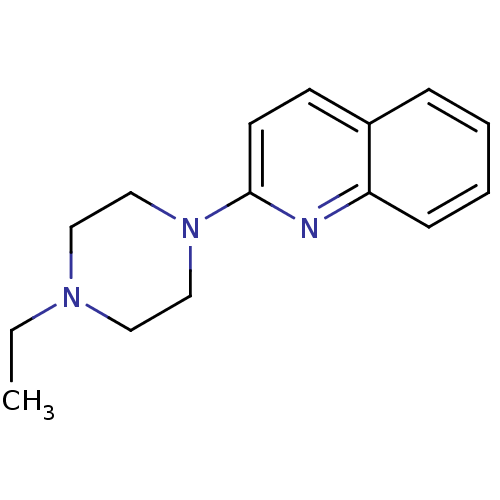
(2-(4-Propyl-piperazin-1-yl)-quinoline | CHEMBL1804...)Show InChI InChI=1S/C15H19N3/c1-2-17-9-11-18(12-10-17)15-8-7-13-5-3-4-6-14(13)16-15/h3-8H,2,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159108
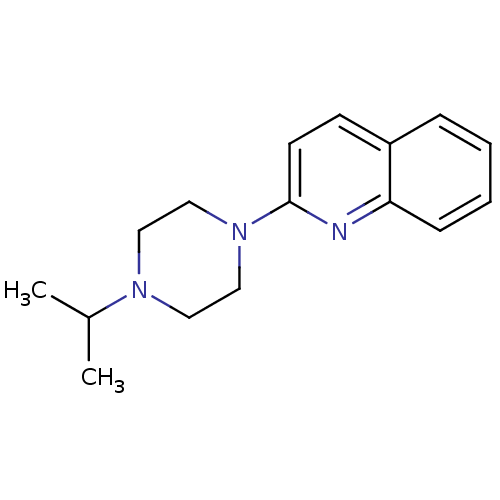
(2-(4-Isopropyl-piperazin-1-yl)-quinoline | CHEMBL3...)Show InChI InChI=1S/C16H21N3/c1-13(2)18-9-11-19(12-10-18)16-8-7-14-5-3-4-6-15(14)17-16/h3-8,13H,9-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159109
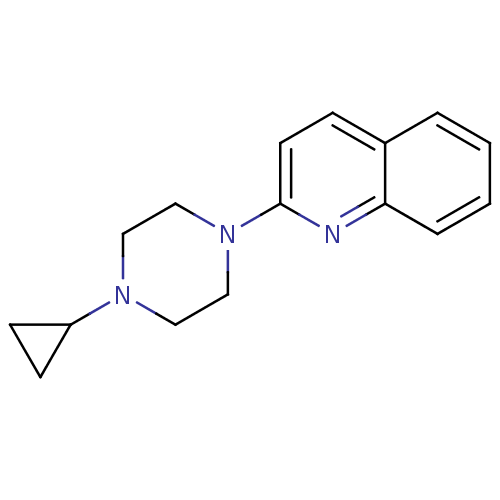
(2-(4-Cyclopropyl-piperazin-1-yl)-quinoline | CHEMB...)Show InChI InChI=1S/C16H19N3/c1-2-4-15-13(3-1)5-8-16(17-15)19-11-9-18(10-12-19)14-6-7-14/h1-5,8,14H,6-7,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159111
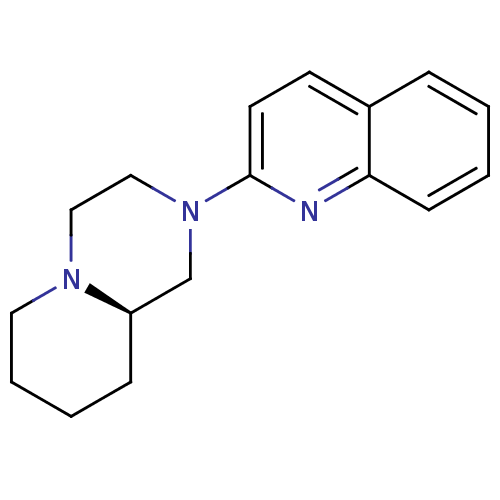
((R)-2-Quinolin-2-yl-octahydro-pyrido[1,2-a]pyrazin...)Show InChI InChI=1S/C17H21N3/c1-2-7-16-14(5-1)8-9-17(18-16)20-12-11-19-10-4-3-6-15(19)13-20/h1-2,5,7-9,15H,3-4,6,10-13H2/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159118
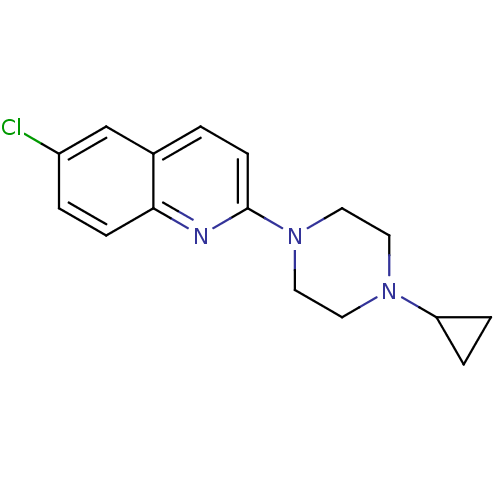
(6-Chloro-2-(4-cyclopropyl-piperazin-1-yl)-quinolin...)Show InChI InChI=1S/C16H18ClN3/c17-13-2-5-15-12(11-13)1-6-16(18-15)20-9-7-19(8-10-20)14-3-4-14/h1-2,5-6,11,14H,3-4,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159117
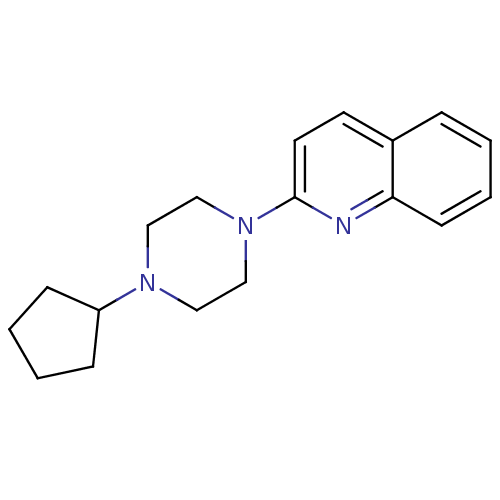
(2-(4-Cyclopentyl-piperazin-1-yl)-quinoline | CHEMB...)Show InChI InChI=1S/C18H23N3/c1-4-8-17-15(5-1)9-10-18(19-17)21-13-11-20(12-14-21)16-6-2-3-7-16/h1,4-5,8-10,16H,2-3,6-7,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159123
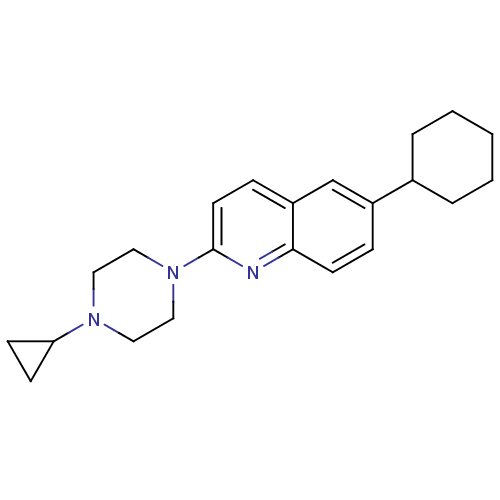
(6-Cyclohexyl-2-(4-cyclopropyl-piperazin-1-yl)-quin...)Show InChI InChI=1S/C22H29N3/c1-2-4-17(5-3-1)18-6-10-21-19(16-18)7-11-22(23-21)25-14-12-24(13-15-25)20-8-9-20/h6-7,10-11,16-17,20H,1-5,8-9,12-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159112

(2-(4-Cyclopropyl-piperazin-1-yl)-6-trifluoromethyl...)Show InChI InChI=1S/C17H18F3N3/c18-17(19,20)13-2-5-15-12(11-13)1-6-16(21-15)23-9-7-22(8-10-23)14-3-4-14/h1-2,5-6,11,14H,3-4,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159115
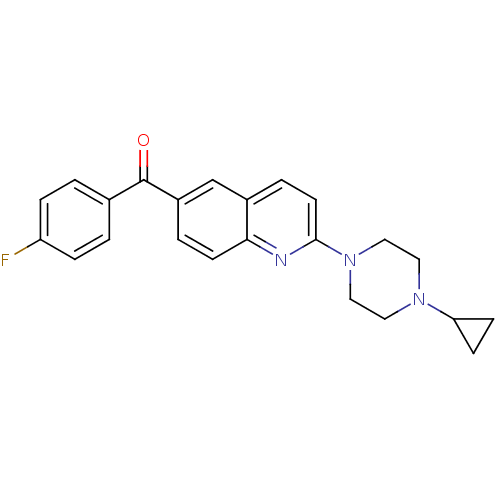
(CHEMBL179306 | [2-(4-Cyclopropyl-piperazin-1-yl)-q...)Show SMILES Fc1ccc(cc1)C(=O)c1ccc2nc(ccc2c1)N1CCN(CC1)C1CC1 Show InChI InChI=1S/C23H22FN3O/c24-19-5-1-16(2-6-19)23(28)18-3-9-21-17(15-18)4-10-22(25-21)27-13-11-26(12-14-27)20-7-8-20/h1-6,9-10,15,20H,7-8,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159124
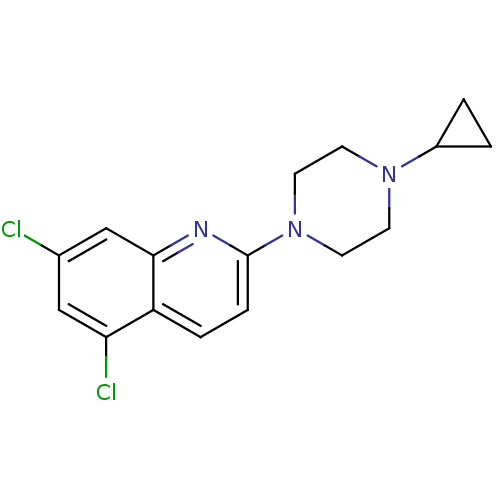
(5,7-Dichloro-2-(4-cyclopropyl-piperazin-1-yl)-quin...)Show InChI InChI=1S/C16H17Cl2N3/c17-11-9-14(18)13-3-4-16(19-15(13)10-11)21-7-5-20(6-8-21)12-1-2-12/h3-4,9-10,12H,1-2,5-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159119
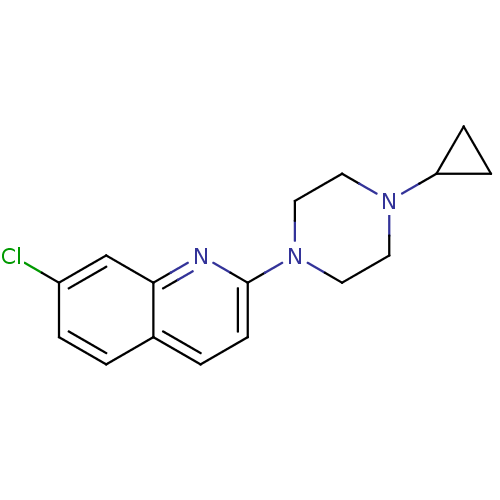
(7-Chloro-2-(4-cyclopropyl-piperazin-1-yl)-quinolin...)Show InChI InChI=1S/C16H18ClN3/c17-13-3-1-12-2-6-16(18-15(12)11-13)20-9-7-19(8-10-20)14-4-5-14/h1-3,6,11,14H,4-5,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 323 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50053631
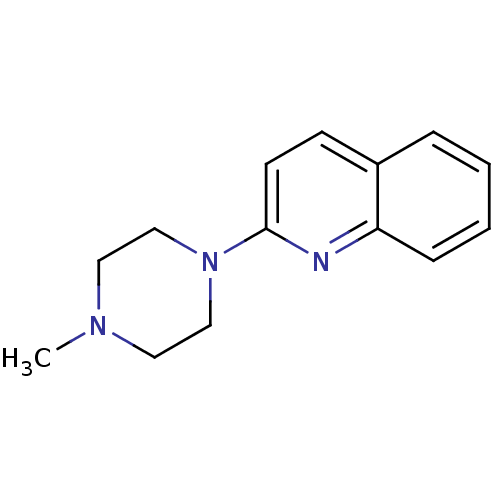
(2-(4-Methyl-piperazin-1-yl)-quinoline | 2-(4-methy...)Show InChI InChI=1S/C14H17N3/c1-16-8-10-17(11-9-16)14-7-6-12-4-2-3-5-13(12)15-14/h2-7H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Antagonist potency against human H3 receptor in GTPgamma[S]-Assay |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50159114

(CHEMBL179234 | Cyclopropyl-[2-(4-cyclopropyl-piper...)Show InChI InChI=1S/C20H23N3O/c24-20(14-1-2-14)16-3-7-18-15(13-16)4-8-19(21-18)23-11-9-22(10-12-23)17-5-6-17/h3-4,7-8,13-14,17H,1-2,5-6,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-aminopotentidine binding to human H2 histamine receptor |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50159114

(CHEMBL179234 | Cyclopropyl-[2-(4-cyclopropyl-piper...)Show InChI InChI=1S/C20H23N3O/c24-20(14-1-2-14)16-3-7-18-15(13-16)4-8-19(21-18)23-11-9-22(10-12-23)17-5-6-17/h3-4,7-8,13-14,17H,1-2,5-6,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-pyrilamine binding to human H1 histamine receptor |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50159114

(CHEMBL179234 | Cyclopropyl-[2-(4-cyclopropyl-piper...)Show InChI InChI=1S/C20H23N3O/c24-20(14-1-2-14)16-3-7-18-15(13-16)4-8-19(21-18)23-11-9-22(10-12-23)17-5-6-17/h3-4,7-8,13-14,17H,1-2,5-6,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibiton of [3H]-histamine binding to human H4 histamine receptor |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50159109

(2-(4-Cyclopropyl-piperazin-1-yl)-quinoline | CHEMB...)Show InChI InChI=1S/C16H19N3/c1-2-4-15-13(3-1)5-8-16(17-15)19-11-9-18(10-12-19)14-6-7-14/h1-5,8,14H,6-7,9-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibiton of [3H]-ketanserin binding to human 5-HT2 receptor |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50159114

(CHEMBL179234 | Cyclopropyl-[2-(4-cyclopropyl-piper...)Show InChI InChI=1S/C20H23N3O/c24-20(14-1-2-14)16-3-7-18-15(13-16)4-8-19(21-18)23-11-9-22(10-12-23)17-5-6-17/h3-4,7-8,13-14,17H,1-2,5-6,9-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Inhibiton of [3H]-ketanserin binding to human 5-HT2 receptor |
J Med Chem 48: 306-11 (2005)
Article DOI: 10.1021/jm040873u
BindingDB Entry DOI: 10.7270/Q2N0161T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data