

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8586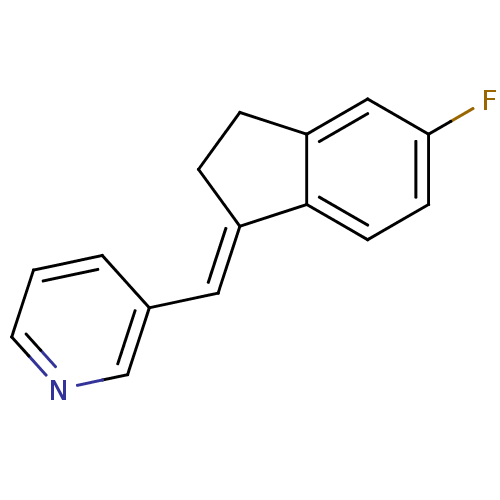 ((3-Pyridylmethylene)indane 5a | 3-{[(1E)-5-fluoro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8608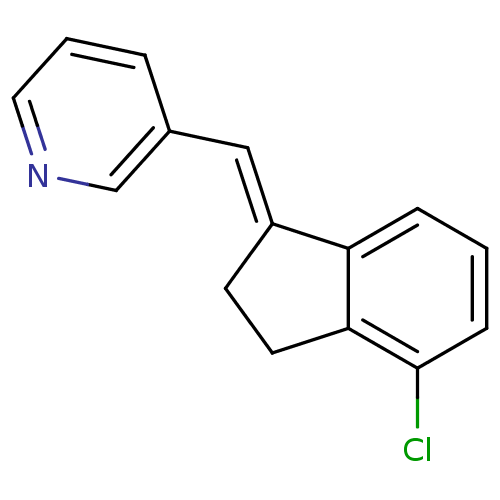 ((3-Pyridylmethylene)indane 25a | 3-{[(1E)-4-chloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8581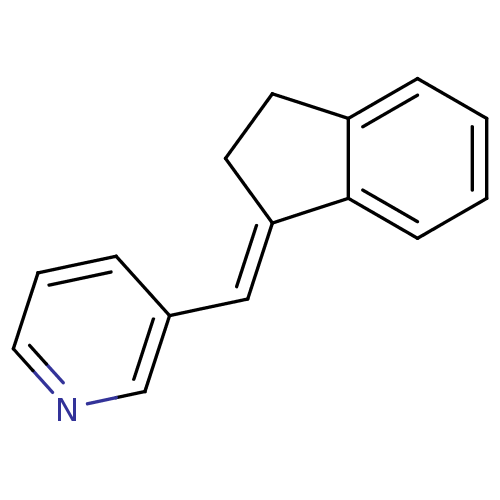 ((3-Pyridylmethylene)indane 1a | 3-[(1E)-2,3-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8587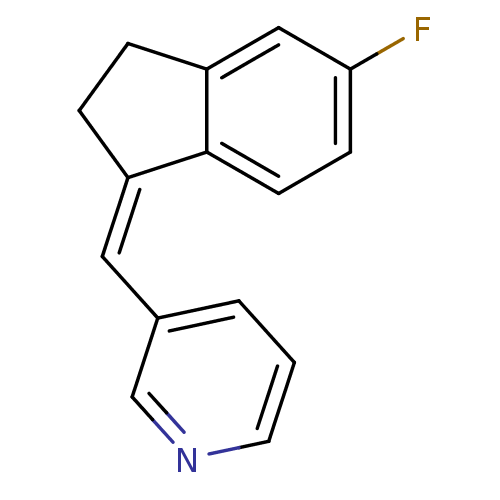 ((3-Pyridylmethylene)indane 5b | 3-{[(1Z)-5-fluoro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8607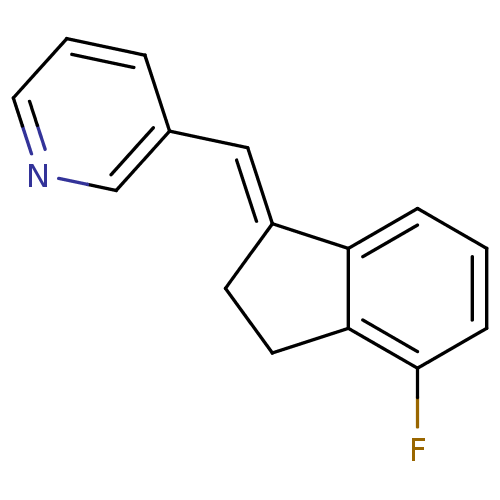 ((3-Pyridylmethylene)indane 24a | 3-{[(1E)-4-fluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8584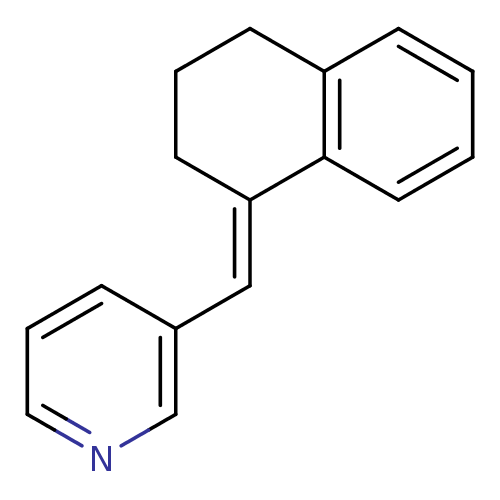 ((3-Pyridylmethylene)tetrahydronaphthalene 3a | 3-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8588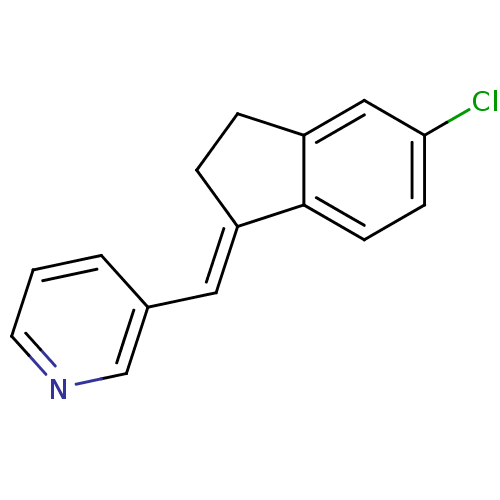 ((3-Pyridylmethylene)indane 7a | 3-{[(1E)-5-chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8640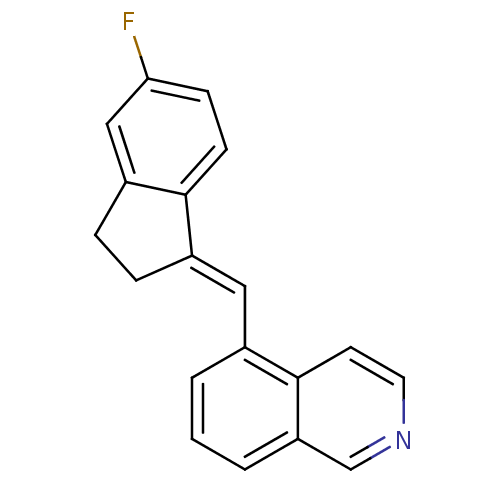 ((isoquinolinemethylene)indane 30b | 5-{[(1Z)-5-flu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8594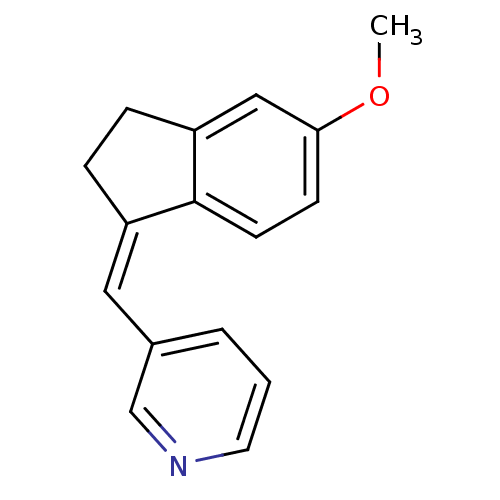 ((3-Pyridylmethylene)indane 11b | 3-{[(1Z)-5-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8631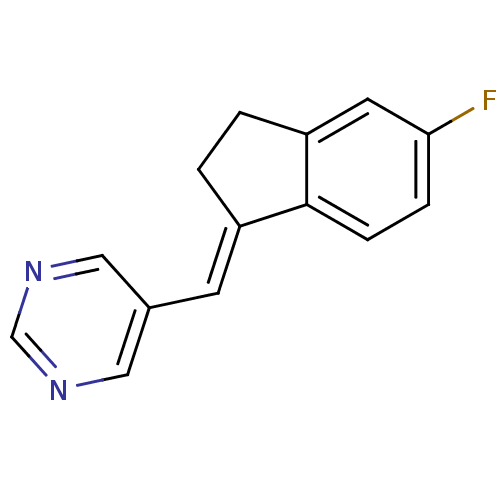 ((5-pyrimidylmethylene)indane 28a | 5-{[(1E)-5-fluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8600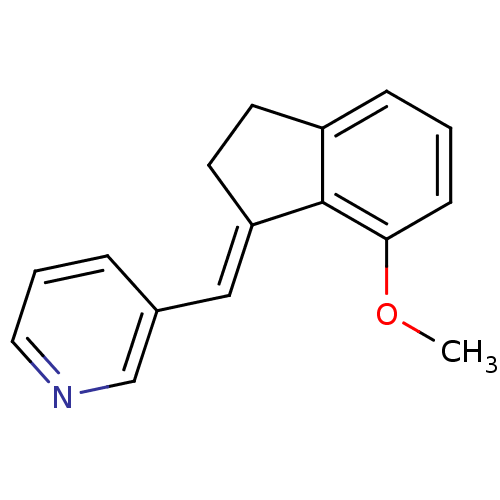 ((3-Pyridylmethylene)indane 26a | 3-{[(1E)-7-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8609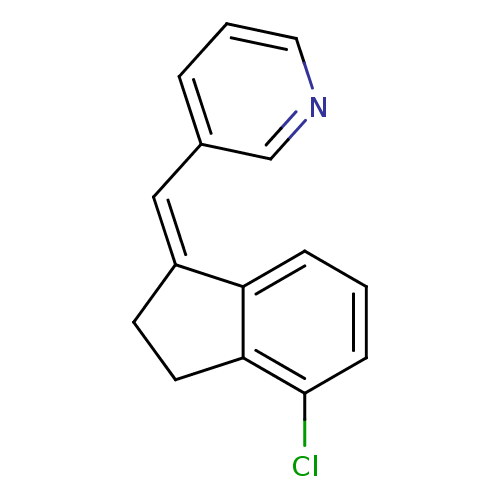 ((3-Pyridylmethylene)indane 25b | 3-{[(1Z)-4-chloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8615 ((4-Pyridylmethylene)indane 6b | 4-{[(1Z)-5-fluoro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8593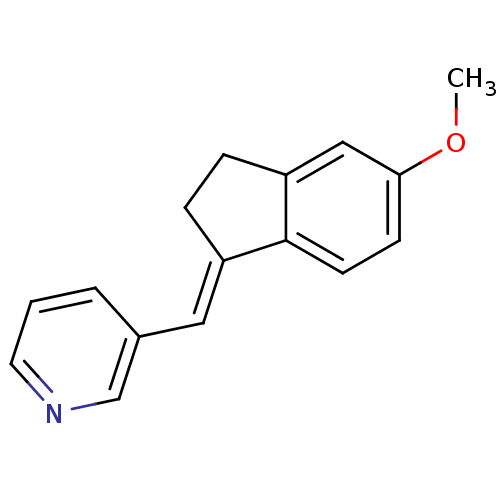 ((3-Pyridylmethylene)indane 11a | 3-{[(1E)-5-methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8590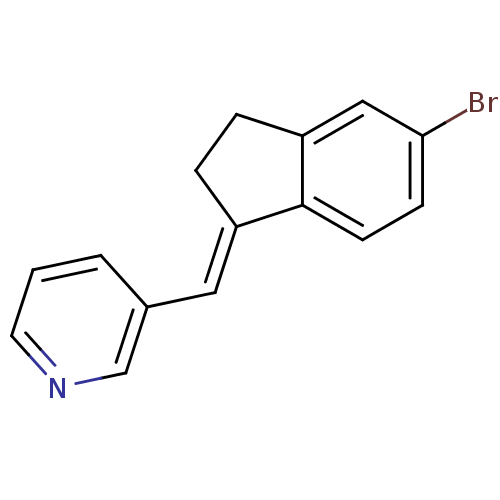 ((3-Pyridylmethylene)indane 9a | 3-{[(1E)-5-bromo-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8595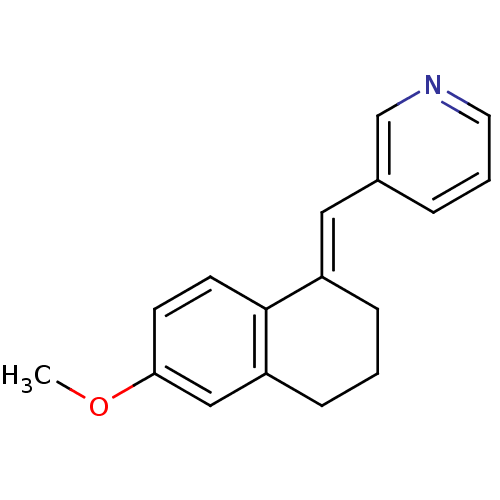 ((3-Pyridylmethylene)tetrahydronaphthalene 13a | 3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8639 ((isoquinolinemethylene)indane 30a | 5-{[(1E)-5-flu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8589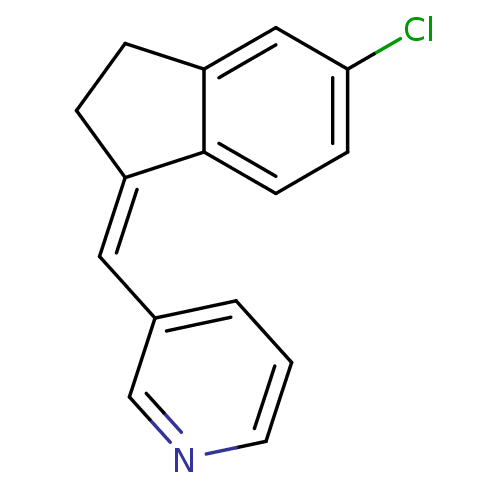 ((3-Pyridylmethylene)indane 7b | 3-{[(1Z)-5-chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8597 ((3-Pyridylmethylene)indane 19a | 3-{[(1E)-5-ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8583 ((3-Pyridylmethylene)indane 1b | 3-[(1Z)-2,3-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8628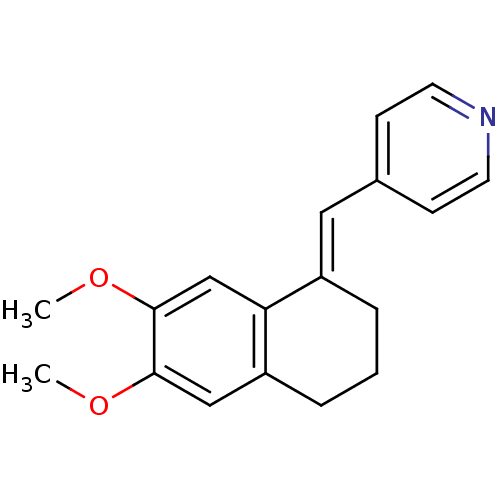 ((4-Pyridylmethylene)tetrahydronaphthalene 18a | 4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8583 ((3-Pyridylmethylene)indane 1b | 3-[(1Z)-2,3-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8645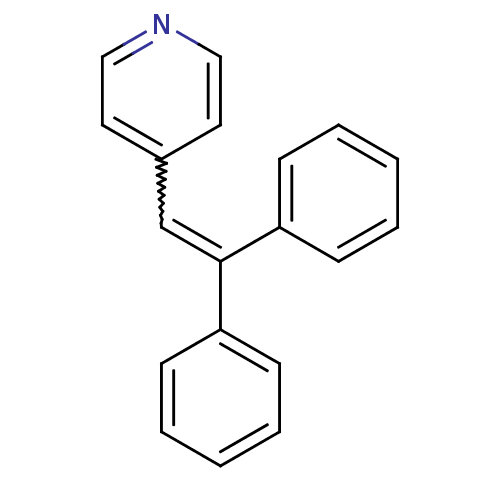 (4-(2,2-diphenylethenyl)pyridine | 4-Pyridylmethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8599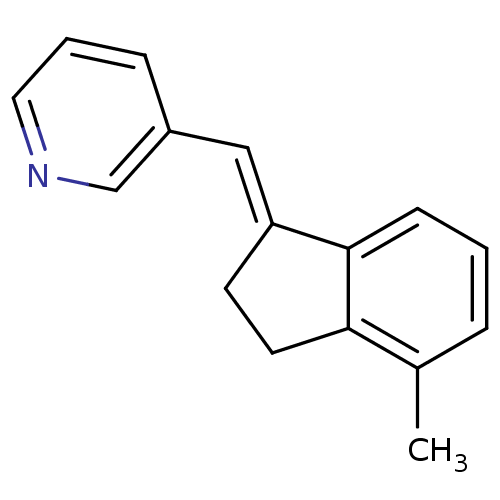 ((3-Pyridylmethylene)indane 23a | 3-{[(1E)-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8587 ((3-Pyridylmethylene)indane 5b | 3-{[(1Z)-5-fluoro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8608 ((3-Pyridylmethylene)indane 25a | 3-{[(1E)-4-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8585 ((3-Pyridylmethylene)tetrahydronaphthalene 3b | 3-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8613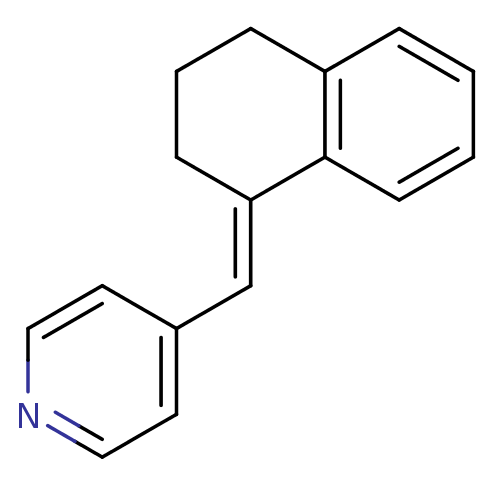 ((4-Pyridylmethylene)tetrahydronaphthalene 4a | 4-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8641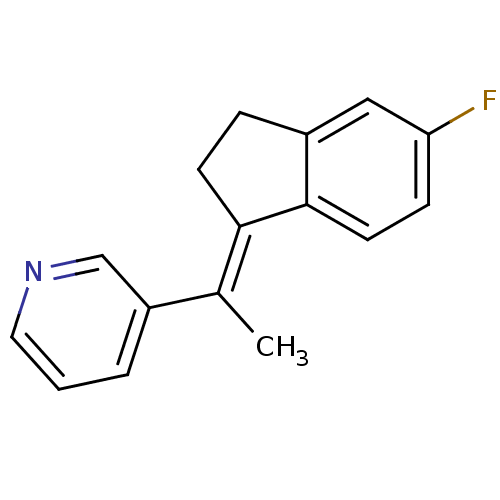 ((3-Pyridylmethylene)indane 38a | 3-{1-[(1E)-5-fluo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8591 ((3-Pyridylmethylene)indane 9b | 3-{[(1Z)-5-bromo-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 171 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8617 ((3-Pyridylmethylene)indane 24b | 3-{[(1Z)-4-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8596 ((3-Pyridylmethylene)tetrahydronaphthalene 13b | 3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8609 ((3-Pyridylmethylene)indane 25b | 3-{[(1Z)-4-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8618 ((4-Pyridylmethylene)indane 8a | 4-{[(1E)-5-chloro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8598 ((3-Pyridylmethylene)indane 19b | 3-{[(1Z)-5-ethoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8615 ((4-Pyridylmethylene)indane 6b | 4-{[(1Z)-5-fluoro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8589 ((3-Pyridylmethylene)indane 7b | 3-{[(1Z)-5-chloro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8626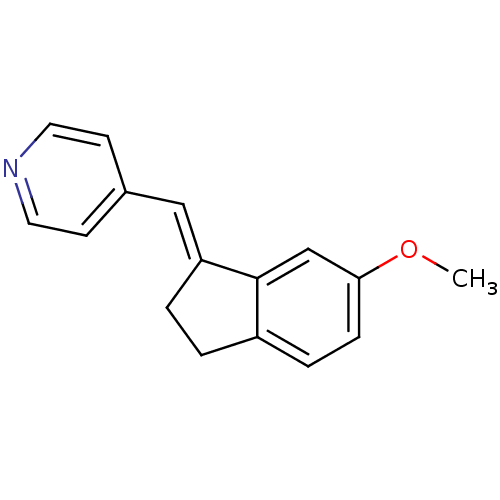 ((4-Pyridylmethylene)indane 16a | 4-{[(1E)-6-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8613 ((4-Pyridylmethylene)tetrahydronaphthalene 4a | 4-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8619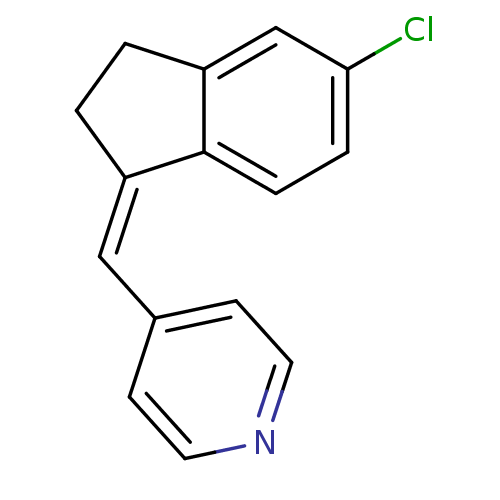 ((4-Pyridylmethylene)indane 8b | 4-{[(1Z)-5-chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 301 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8608 ((3-Pyridylmethylene)indane 25a | 3-{[(1E)-4-chloro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8586 ((3-Pyridylmethylene)indane 5a | 3-{[(1E)-5-fluoro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 311 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8591 ((3-Pyridylmethylene)indane 9b | 3-{[(1Z)-5-bromo-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8640 ((isoquinolinemethylene)indane 30b | 5-{[(1Z)-5-flu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 374 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8614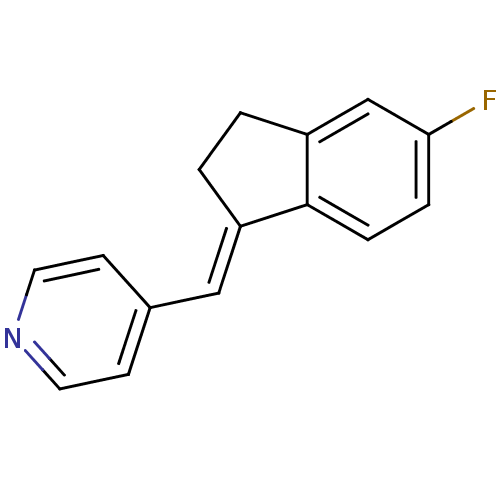 ((4-Pyridylmethylene)indane 6a | 4-{[(1E)-5-fluoro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8621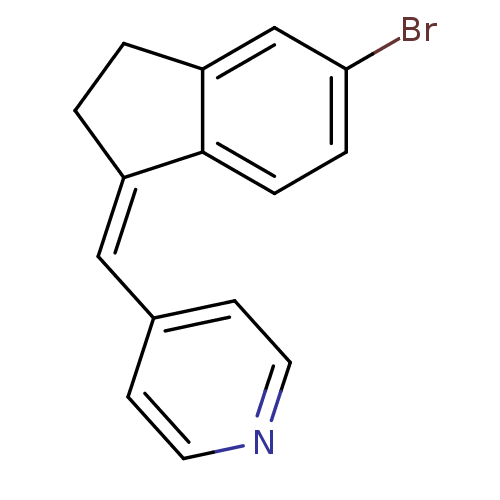 ((4-Pyridylmethylene)indane 10b | 4-{[(1Z)-5-bromo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8613 ((4-Pyridylmethylene)tetrahydronaphthalene 4a | 4-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8609 ((3-Pyridylmethylene)indane 25b | 3-{[(1Z)-4-chloro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 657 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8584 ((3-Pyridylmethylene)tetrahydronaphthalene 3a | 3-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 715 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8639 ((isoquinolinemethylene)indane 30a | 5-{[(1E)-5-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8599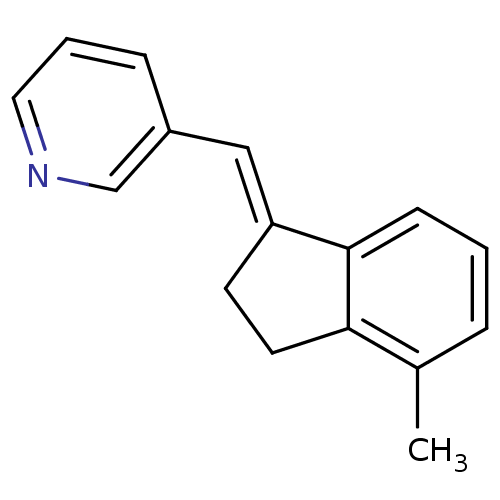 ((3-Pyridylmethylene)indane 23a | 3-{[(1E)-4-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 763 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8627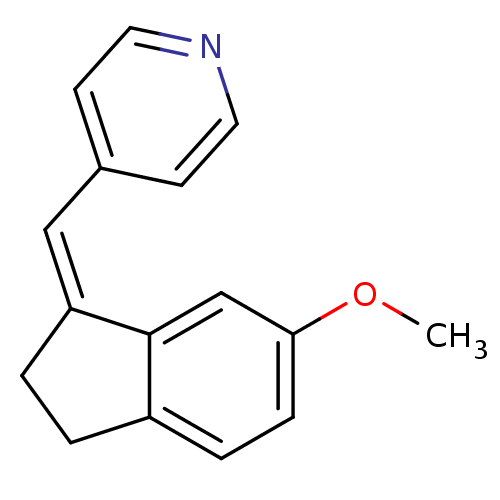 ((4-Pyridylmethylene)indane 16b | 4-{[(1Z)-6-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8607 ((3-Pyridylmethylene)indane 24a | 3-{[(1E)-4-fluoro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 774 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8634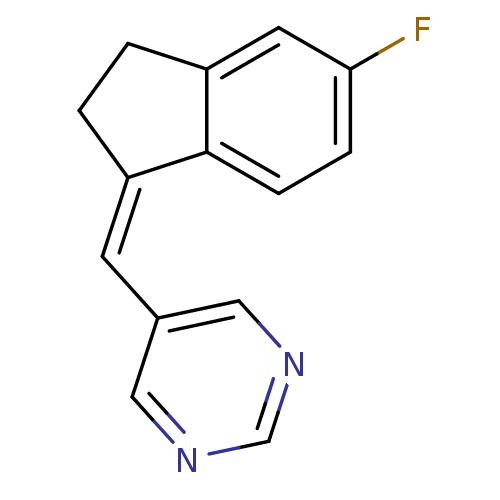 ((5-pyrimidylmethylene)indane 28b | 5-{[(1Z)-5-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8594 ((3-Pyridylmethylene)indane 11b | 3-{[(1Z)-5-methox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8615 ((4-Pyridylmethylene)indane 6b | 4-{[(1Z)-5-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8624 ((4-Pyridylmethylene)tetrahydronaphthalene 14a | 4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8621 ((4-Pyridylmethylene)indane 10b | 4-{[(1Z)-5-bromo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 877 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8596 ((3-Pyridylmethylene)tetrahydronaphthalene 13b | 3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 878 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8581 ((3-Pyridylmethylene)indane 1a | 3-[(1E)-2,3-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 888 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8595 ((3-Pyridylmethylene)tetrahydronaphthalene 13a | 3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 903 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8612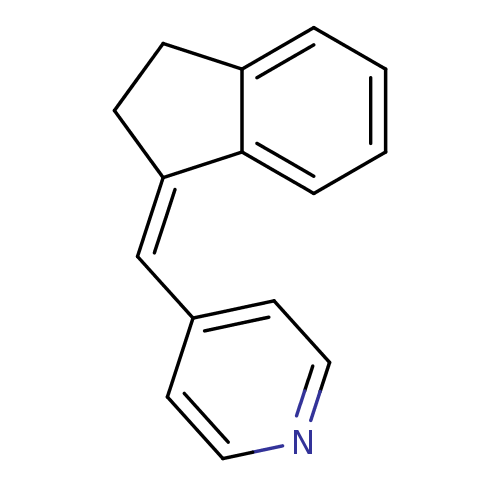 ((4-Pyridylmethylene)indane 2b | 4-[(1Z)-2,3-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 931 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8620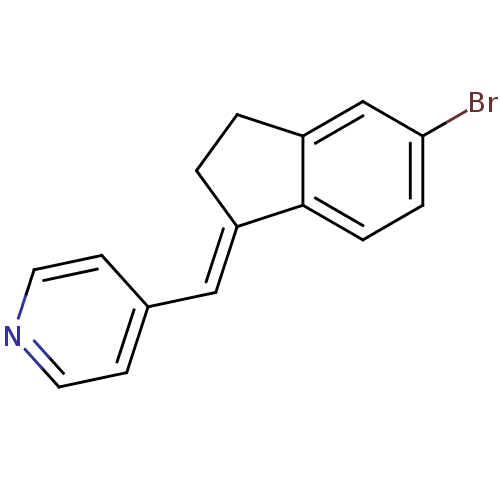 ((4-Pyridylmethylene)indane 10a | 4-{[(1E)-5-bromo-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 948 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8600 ((3-Pyridylmethylene)indane 26a | 3-{[(1E)-7-methox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 955 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8602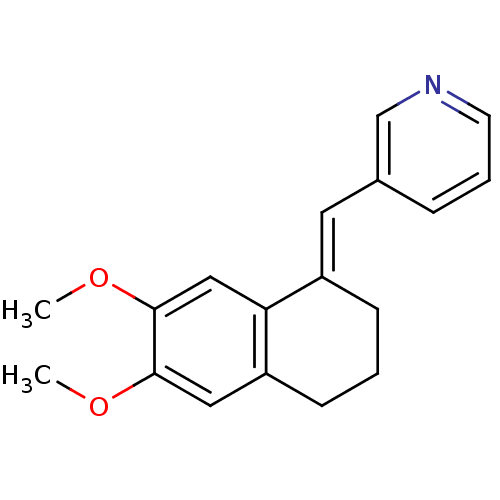 ((3-Pyridylmethylene)tetrahydronaphthalene 17a | 3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8625 ((4-Pyridylmethylene)tetrahydronaphthalene 14b | 4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8614 ((4-Pyridylmethylene)indane 6a | 4-{[(1E)-5-fluoro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8619 ((4-Pyridylmethylene)indane 8b | 4-{[(1Z)-5-chloro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8639 ((isoquinolinemethylene)indane 30a | 5-{[(1E)-5-flu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8595 ((3-Pyridylmethylene)tetrahydronaphthalene 13a | 3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8629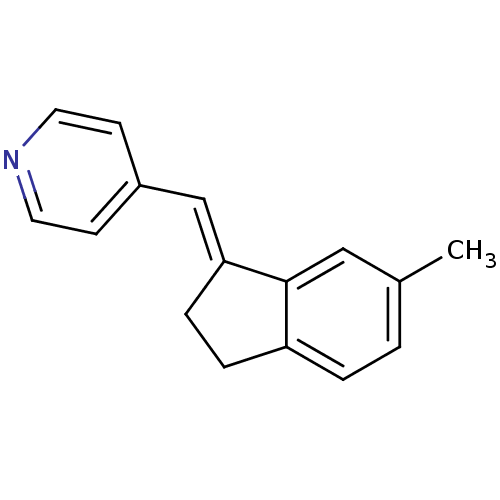 ((4-Pyridylmethylene)indane 22a | 4-{[(1E)-6-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8612 ((4-Pyridylmethylene)indane 2b | 4-[(1Z)-2,3-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8585 ((3-Pyridylmethylene)tetrahydronaphthalene 3b | 3-[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8593 ((3-Pyridylmethylene)indane 11a | 3-{[(1E)-5-methox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8588 ((3-Pyridylmethylene)indane 7a | 3-{[(1E)-5-chloro-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8618 ((4-Pyridylmethylene)indane 8a | 4-{[(1E)-5-chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8614 ((4-Pyridylmethylene)indane 6a | 4-{[(1E)-5-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8630 ((4-Pyridylmethylene)indane 22b | 4-{[(1Z)-6-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8607 ((3-Pyridylmethylene)indane 24a | 3-{[(1E)-4-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8590 ((3-Pyridylmethylene)indane 9a | 3-{[(1E)-5-bromo-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8640 ((isoquinolinemethylene)indane 30b | 5-{[(1Z)-5-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8619 ((4-Pyridylmethylene)indane 8b | 4-{[(1Z)-5-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8581 ((3-Pyridylmethylene)indane 1a | 3-[(1E)-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8597 ((3-Pyridylmethylene)indane 19a | 3-{[(1E)-5-ethoxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8587 ((3-Pyridylmethylene)indane 5b | 3-{[(1Z)-5-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8586 ((3-Pyridylmethylene)indane 5a | 3-{[(1E)-5-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.54E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8620 ((4-Pyridylmethylene)indane 10a | 4-{[(1E)-5-bromo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8598 ((3-Pyridylmethylene)indane 19b | 3-{[(1Z)-5-ethoxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8644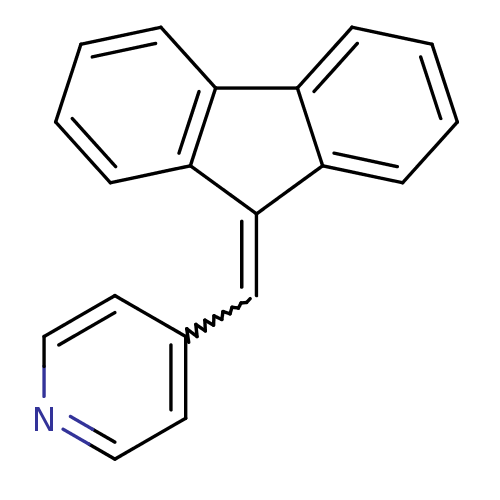 ((4-Pyridylmethylene)fluorene 34 | 4-(9H-fluoren-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8647 ((3-Pyridylmethylene)indane 36b | 3-{[(1Z)-3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8631 ((5-pyrimidylmethylene)indane 28a | 5-{[(1E)-5-fluo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8621 ((4-Pyridylmethylene)indane 10b | 4-{[(1Z)-5-bromo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8583 ((3-Pyridylmethylene)indane 1b | 3-[(1Z)-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.55E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8638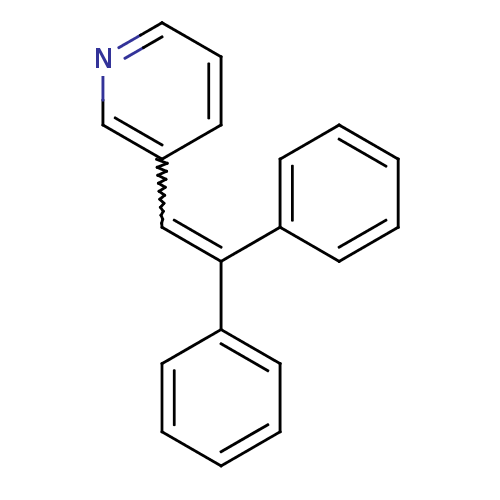 (3-(2,2-diphenylethenyl)pyridine | 3-Pyridylmethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8646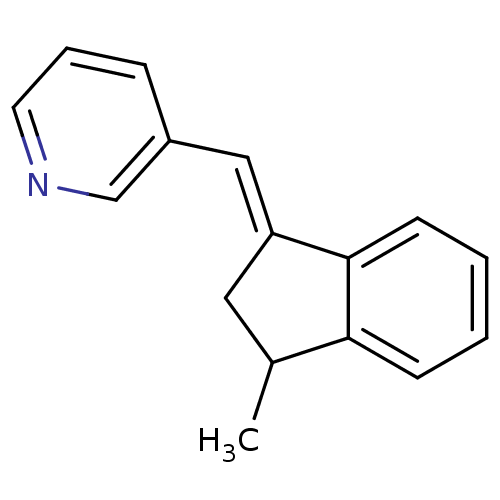 ((3-Pyridylmethylene)indane 36a | 3-{[(1E)-3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8623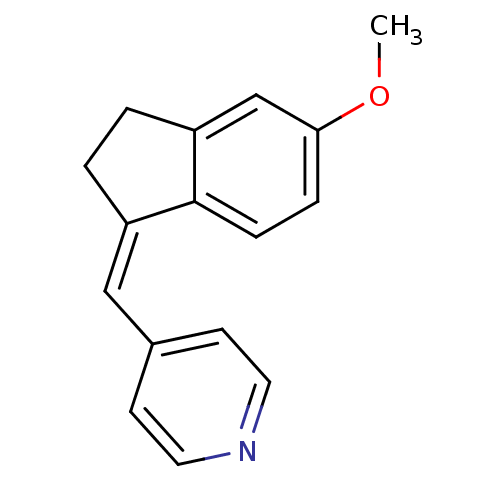 ((4-Pyridylmethylene)indane 12b | 4-{[(1Z)-5-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8585 ((3-Pyridylmethylene)tetrahydronaphthalene 3b | 3-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.79E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8604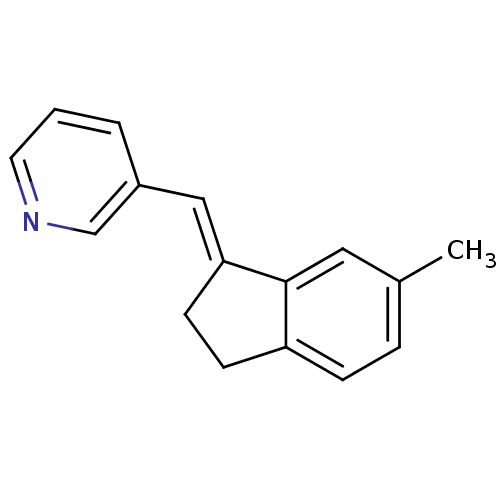 ((3-Pyridylmethylene)indane 21a | 3-{[(1E)-6-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8622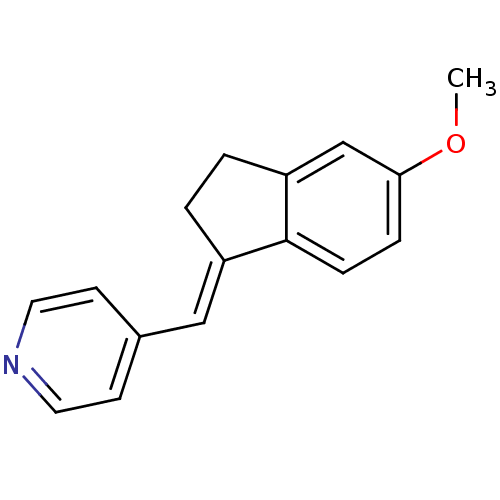 ((4-Pyridylmethylene)indane 12a | 4-{[(1E)-5-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8596 ((3-Pyridylmethylene)tetrahydronaphthalene 13b | 3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8589 ((3-Pyridylmethylene)indane 7b | 3-{[(1Z)-5-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.05E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8648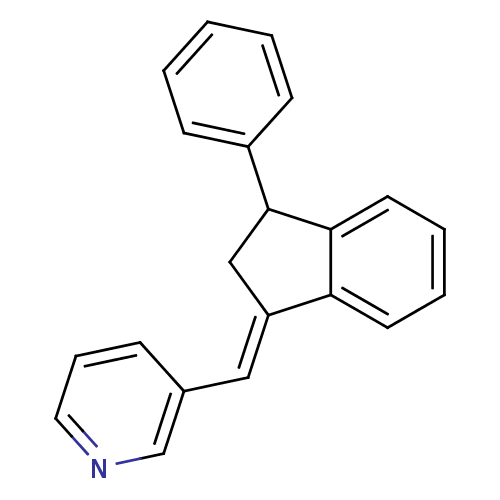 ((3-Pyridylmethylene)indane 37a | 3-{[(1E)-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8588 ((3-Pyridylmethylene)indane 7a | 3-{[(1E)-5-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.26E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8593 ((3-Pyridylmethylene)indane 11a | 3-{[(1E)-5-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8632 ((5-Thiazolemethylene)indane 27a | 5-{[(1E)-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8605 ((3-Pyridylmethylene)indane 21b | 3-{[(1Z)-6-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8599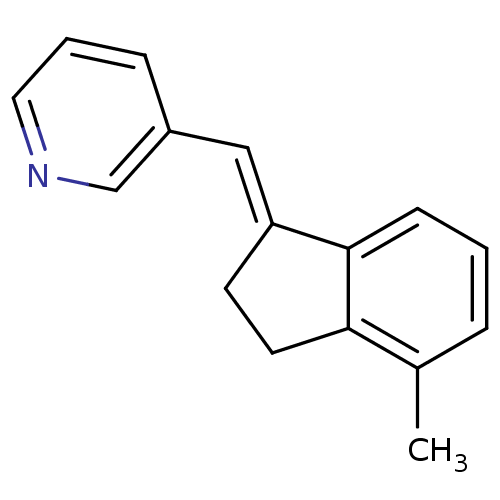 ((3-Pyridylmethylene)indane 23a | 3-{[(1E)-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8637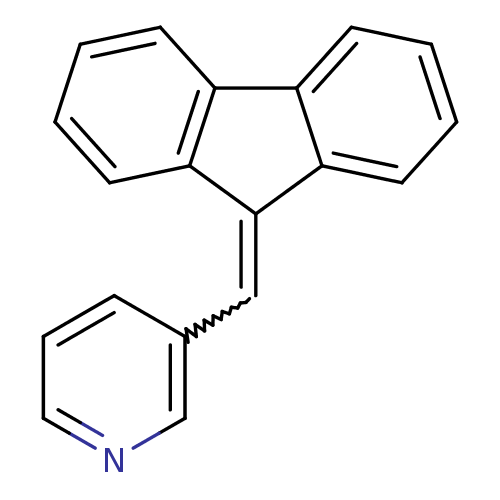 ((3-Pyridylmethylene)fluorene 32 | 3-(9H-fluoren-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8633 ((5-Thiazolemethylene)indane 27b | 5-{[(1Z)-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8618 ((4-Pyridylmethylene)indane 8a | 4-{[(1E)-5-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8594 ((3-Pyridylmethylene)indane 11b | 3-{[(1Z)-5-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8601 ((3-Pyridylmethylene)indane 15a | 3-{[(1E)-6-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8584 ((3-Pyridylmethylene)tetrahydronaphthalene 3a | 3-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.58E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8616 ((4-Pyridylmethylene)indane 2a | 4-[(1E)-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8597 ((3-Pyridylmethylene)indane 19a | 3-{[(1E)-5-ethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8591 ((3-Pyridylmethylene)indane 9b | 3-{[(1Z)-5-bromo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.37E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8631 ((5-pyrimidylmethylene)indane 28a | 5-{[(1E)-5-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8598 ((3-Pyridylmethylene)indane 19b | 3-{[(1Z)-5-ethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8590 ((3-Pyridylmethylene)indane 9a | 3-{[(1E)-5-bromo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.83E+3 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8600 ((3-Pyridylmethylene)indane 26a | 3-{[(1E)-7-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8606 ((3-Pyridylmethylene)indane 23b | 3-{[(1Z)-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8612 ((4-Pyridylmethylene)indane 2b | 4-[(1Z)-2,3-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8603 ((3-Pyridylmethylene)indane 20a | 3-{[(1E)-5-(benzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8620 ((4-Pyridylmethylene)indane 10a | 4-{[(1E)-5-bromo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8635 ((quinolinemethylene)indane 29a | 5-{[(1E)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8643 ((quinolinemethylene)indane 31b | 4-{[(1Z)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8642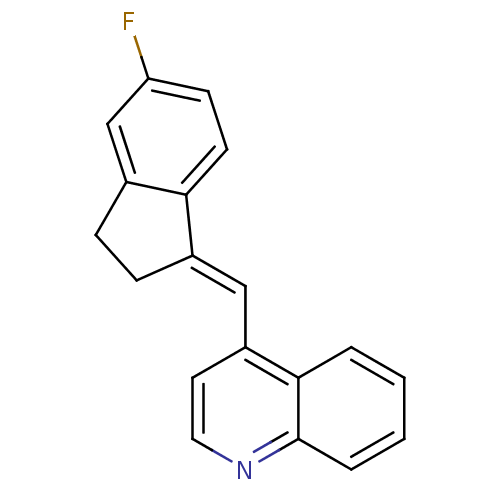 ((quinolinemethylene)indane 31a | 4-{[(1E)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8636 ((quinolinemethylene)indane 29b | 5-{[(1Z)-5-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 48: 1563-75 (2005) Article DOI: 10.1021/jm0492397 BindingDB Entry DOI: 10.7270/Q2SN075F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||