

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162972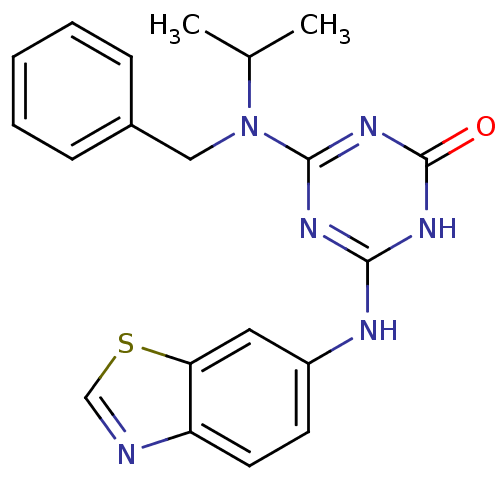 (4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162976 (4-(Benzothiazol-6-ylamino)-6-(1-methyl-1-phenyl-et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162973 (CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162973 (CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162978 (4-(Benzothiazol-6-ylamino)-6-(benzyl-ethyl-amino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50162972 (4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of tyrosine protein kinase receptor TIE-2 | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162975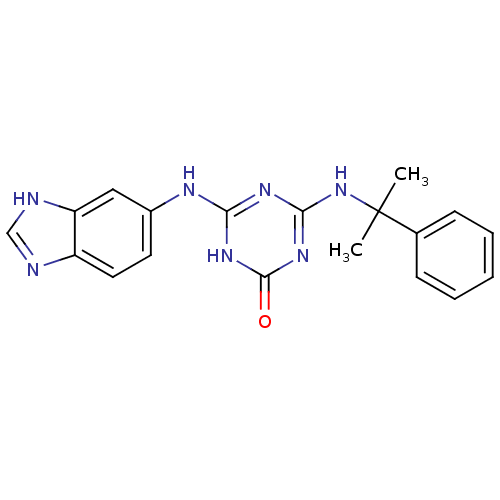 (4-(3H-Benzoimidazol-5-ylamino)-6-(1-methyl-1-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162972 (4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of KDR-induced MAP kinase autophosphorylation assay in HUVEC cells | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162974 (4-(Benzothiazol-5-ylamino)-6-(benzyl-methyl-amino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162974 (4-(Benzothiazol-5-ylamino)-6-(benzyl-methyl-amino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50162978 (4-(Benzothiazol-6-ylamino)-6-(benzyl-ethyl-amino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of tyrosine protein kinase receptor TIE-2 | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162976 (4-(Benzothiazol-6-ylamino)-6-(1-methyl-1-phenyl-et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of KDR-induced MAP kinase autophosphorylation assay in HUVEC cells | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50162973 (CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of beta IRK tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50162976 (4-(Benzothiazol-6-ylamino)-6-(1-methyl-1-phenyl-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of beta IRK tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162978 (4-(Benzothiazol-6-ylamino)-6-(benzyl-ethyl-amino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of KDR-induced MAP kinase autophosphorylation assay in HUVEC cells | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50162973 (CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of tyrosine protein kinase receptor TIE-2 | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiopoietin-1 receptor (Homo sapiens (Human)) | BDBM50162976 (4-(Benzothiazol-6-ylamino)-6-(1-methyl-1-phenyl-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of tyrosine protein kinase receptor TIE-2 | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162977 (4-(Benzothiazol-6-yl-methyl-amino)-6-(1-methyl-1-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162981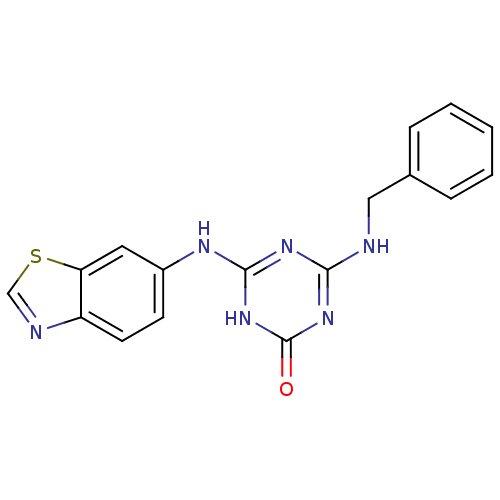 (4-(Benzothiazol-6-ylamino)-6-benzylamino-[1,3,5]tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50162978 (4-(Benzothiazol-6-ylamino)-6-(benzyl-ethyl-amino)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of PDGF-R2 tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50162973 (CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of PDGF-R2 tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50162978 (4-(Benzothiazol-6-ylamino)-6-(benzyl-ethyl-amino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of beta IRK tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50162978 (4-(Benzothiazol-6-ylamino)-6-(benzyl-ethyl-amino)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of c-fms tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162980 (CHEMBL175760 | N-Benzothiazol-6-yl-6-chloro-N''-(1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162980 (CHEMBL175760 | N-Benzothiazol-6-yl-6-chloro-N''-(1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162979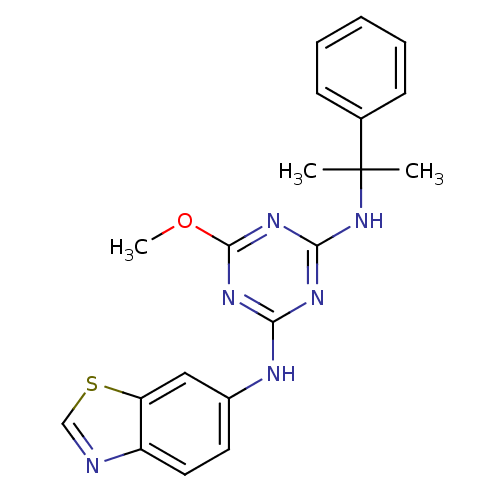 (CHEMBL175506 | N-Benzothiazol-6-yl-6-methoxy-N''-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50162976 (4-(Benzothiazol-6-ylamino)-6-(1-methyl-1-phenyl-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of PDGF-R2 tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162977 (4-(Benzothiazol-6-yl-methyl-amino)-6-(1-methyl-1-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of KDR-induced MAP kinase autophosphorylation assay in HUVEC cells | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Mus musculus) | BDBM50162978 (4-(Benzothiazol-6-ylamino)-6-(benzyl-ethyl-amino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of mouse Fibroblast growth factor receptor 1 expressed in NIH 3T3 fibroblasts | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50162973 (CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human epidermal growth factor receptor | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Mus musculus) | BDBM50162972 (4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of mouse Fibroblast growth factor receptor 1 expressed in NIH 3T3 fibroblasts | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Mus musculus) | BDBM50162976 (4-(Benzothiazol-6-ylamino)-6-(1-methyl-1-phenyl-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of mouse Fibroblast growth factor receptor 1 expressed in NIH 3T3 fibroblasts | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Mus musculus) | BDBM50162973 (CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of mouse Fibroblast growth factor receptor 1 expressed in NIH 3T3 fibroblasts | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50162978 (4-(Benzothiazol-6-ylamino)-6-(benzyl-ethyl-amino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human Insulin receptor expressed in CHO cells | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50162978 (4-(Benzothiazol-6-ylamino)-6-(benzyl-ethyl-amino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human epidermal growth factor receptor | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50162973 (CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human Insulin receptor expressed in CHO cells | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50162972 (4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human epidermal growth factor receptor | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50162976 (4-(Benzothiazol-6-ylamino)-6-(1-methyl-1-phenyl-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human epidermal growth factor receptor | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50162972 (4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human Insulin receptor expressed in CHO cells | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50162976 (4-(Benzothiazol-6-ylamino)-6-(1-methyl-1-phenyl-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human Insulin receptor expressed in CHO cells | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50162972 (4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of beta IRK tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50162972 (4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of c-fms tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50162976 (4-(Benzothiazol-6-ylamino)-6-(1-methyl-1-phenyl-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of c-fms tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha/beta (Homo sapiens (Human)) | BDBM50162972 (4-(Benzothiazol-6-ylamino)-6-(benzyl-isopropyl-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of PDGF-R2 tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50162973 (CHEMBL177298 | N-[4-(Benzothiazol-6-ylamino)-6-(1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of c-fms tyrosine kinase | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162975 (4-(3H-Benzoimidazol-5-ylamino)-6-(1-methyl-1-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of Vascular endothelial growth factor receptor 2 at 10 uM ATP | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162981 (4-(Benzothiazol-6-ylamino)-6-benzylamino-[1,3,5]tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of KDR-induced MAP kinase autophosphorylation assay in HUVEC cells | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50162979 (CHEMBL175506 | N-Benzothiazol-6-yl-6-methoxy-N''-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of KDR-induced MAP kinase autophosphorylation assay in HUVEC cells | J Med Chem 48: 1717-20 (2005) Article DOI: 10.1021/jm049372z BindingDB Entry DOI: 10.7270/Q2GM86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||