

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8871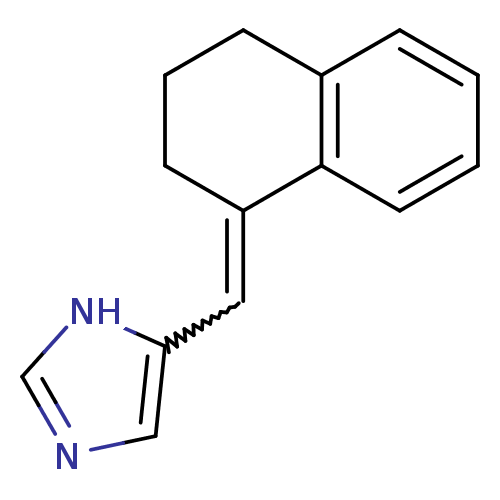 (5-[(1E)-1,2,3,4-tetrahydronaphthalen-1-ylidenemeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8881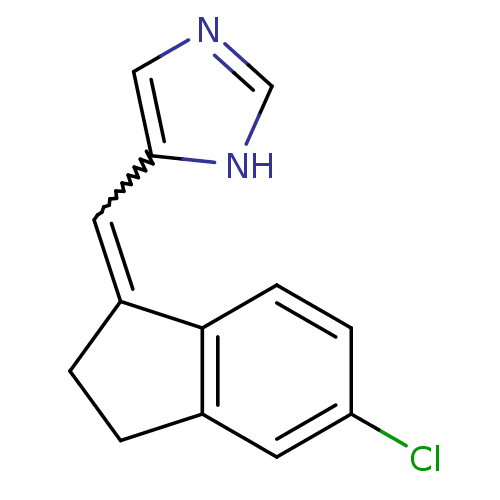 (5-[(E)-(5-Chloro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8873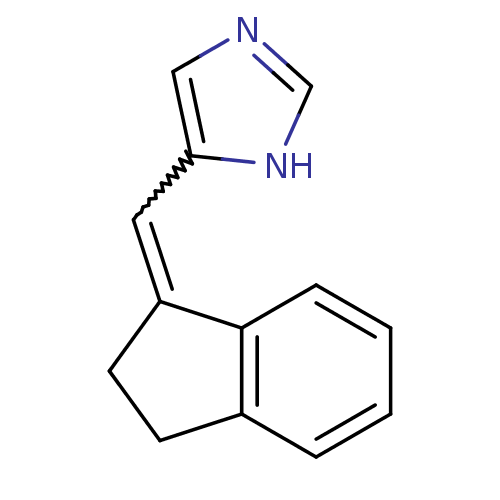 (5-[(1E)-2,3-dihydro-1H-inden-1-ylidenemethyl]-1H-i...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8875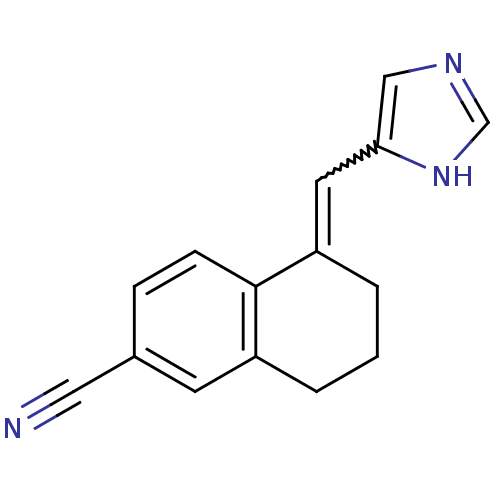 ((5Z)-5-(1H-Imidazol-5-ylmethylene)-5,6,7,8-tetrahy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8871 (5-[(1E)-1,2,3,4-tetrahydronaphthalen-1-ylidenemeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8883 (5-[(E)-(5-Bromo-2,3-dihydro-1H-inden-1-ylidene)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8873 (5-[(1E)-2,3-dihydro-1H-inden-1-ylidenemethyl]-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8879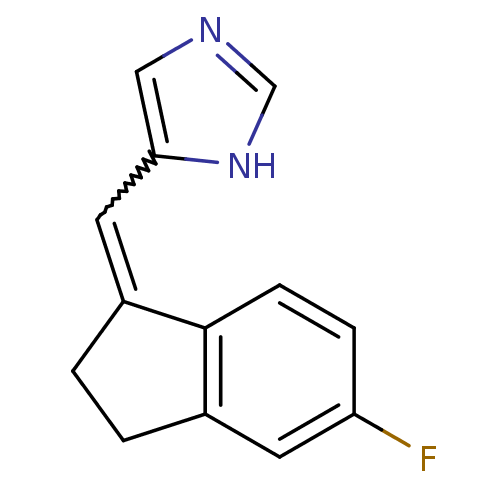 (5-[(E)-(5-Fluoro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.2 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8876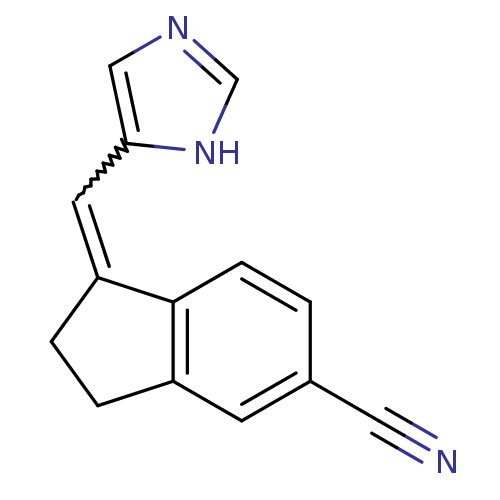 ((1E)-1-(1H-Imidazol-5-ylmethylene)indane-5-carboni...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8879 (5-[(E)-(5-Fluoro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8876 ((1E)-1-(1H-Imidazol-5-ylmethylene)indane-5-carboni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8876 ((1E)-1-(1H-Imidazol-5-ylmethylene)indane-5-carboni...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8879 (5-[(E)-(5-Fluoro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8878 (5-[(E)-(7-Chloro-3,4-dihydronaphthalen-1(2H)-ylide...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.7 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8881 (5-[(E)-(5-Chloro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8879 (5-[(E)-(5-Fluoro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8879 (5-[(E)-(5-Fluoro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.6 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8875 ((5Z)-5-(1H-Imidazol-5-ylmethylene)-5,6,7,8-tetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8883 (5-[(E)-(5-Bromo-2,3-dihydro-1H-inden-1-ylidene)met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8871 (5-[(1E)-1,2,3,4-tetrahydronaphthalen-1-ylidenemeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8873 (5-[(1E)-2,3-dihydro-1H-inden-1-ylidenemethyl]-1H-i...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25.9 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8883 (5-[(E)-(5-Bromo-2,3-dihydro-1H-inden-1-ylidene)met...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26.2 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8883 (5-[(E)-(5-Bromo-2,3-dihydro-1H-inden-1-ylidene)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8881 (5-[(E)-(5-Chloro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28.7 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8871 (5-[(1E)-1,2,3,4-tetrahydronaphthalen-1-ylidenemeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31.4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8876 ((1E)-1-(1H-Imidazol-5-ylmethylene)indane-5-carboni...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8876 ((1E)-1-(1H-Imidazol-5-ylmethylene)indane-5-carboni...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8881 (5-[(E)-(5-Chloro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8873 (5-[(1E)-2,3-dihydro-1H-inden-1-ylidenemethyl]-1H-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8878 (5-[(E)-(7-Chloro-3,4-dihydronaphthalen-1(2H)-ylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8881 (5-[(E)-(5-Chloro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8883 (5-[(E)-(5-Bromo-2,3-dihydro-1H-inden-1-ylidene)met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8883 (5-[(E)-(5-Bromo-2,3-dihydro-1H-inden-1-ylidene)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8876 ((1E)-1-(1H-Imidazol-5-ylmethylene)indane-5-carboni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8878 (5-[(E)-(7-Chloro-3,4-dihydronaphthalen-1(2H)-ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8875 ((5Z)-5-(1H-Imidazol-5-ylmethylene)-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8873 (5-[(1E)-2,3-dihydro-1H-inden-1-ylidenemethyl]-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8871 (5-[(1E)-1,2,3,4-tetrahydronaphthalen-1-ylidenemeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8879 (5-[(E)-(5-Fluoro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8871 (5-[(1E)-1,2,3,4-tetrahydronaphthalen-1-ylidenemeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8881 (5-[(E)-(5-Chloro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8886 ((8E)-8-(1H-Imidazol-5-ylmethylene)-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8873 (5-[(1E)-2,3-dihydro-1H-inden-1-ylidenemethyl]-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 955 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8886 ((8E)-8-(1H-Imidazol-5-ylmethylene)-5,6,7,8-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8881 (5-[(E)-(5-Chloro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8881 (5-[(E)-(5-Chloro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8875 ((5Z)-5-(1H-Imidazol-5-ylmethylene)-5,6,7,8-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8878 (5-[(E)-(7-Chloro-3,4-dihydronaphthalen-1(2H)-ylide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8879 (5-[(E)-(5-Fluoro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8883 (5-[(E)-(5-Bromo-2,3-dihydro-1H-inden-1-ylidene)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8871 (5-[(1E)-1,2,3,4-tetrahydronaphthalen-1-ylidenemeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8873 (5-[(1E)-2,3-dihydro-1H-inden-1-ylidenemethyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8876 ((1E)-1-(1H-Imidazol-5-ylmethylene)indane-5-carboni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8883 (5-[(E)-(5-Bromo-2,3-dihydro-1H-inden-1-ylidene)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8886 ((8E)-8-(1H-Imidazol-5-ylmethylene)-5,6,7,8-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8876 ((1E)-1-(1H-Imidazol-5-ylmethylene)indane-5-carboni...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8871 (5-[(1E)-1,2,3,4-tetrahydronaphthalen-1-ylidenemeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8886 ((8E)-8-(1H-Imidazol-5-ylmethylene)-5,6,7,8-tetrahy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8879 (5-[(E)-(5-Fluoro-2,3-dihydro-1H-inden-1-ylidene)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8873 (5-[(1E)-2,3-dihydro-1H-inden-1-ylidenemethyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 48: 1796-805 (2005) Article DOI: 10.1021/jm049600p BindingDB Entry DOI: 10.7270/Q2RN362H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||