 Found 125 hits of Enzyme Inhibition Constant Data
Found 125 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 3
(Rattus norvegicus) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of rat eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163650

(1-(3,5-Diacetyl-phenyl)-3-{(1R,2S)-2-[(S)-3-(4-flu...)Show SMILES CC(=O)c1cc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc(c1)C(C)=O Show InChI InChI=1S/C30H38FN3O3/c1-20(35)25-15-26(21(2)36)17-28(16-25)32-30(37)33-29-8-4-3-7-24(29)19-34-13-5-6-23(18-34)14-22-9-11-27(31)12-10-22/h9-12,15-17,23-24,29H,3-8,13-14,18-19H2,1-2H3,(H2,32,33,37)/t23-,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Rattus norvegicus) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of rat eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163661
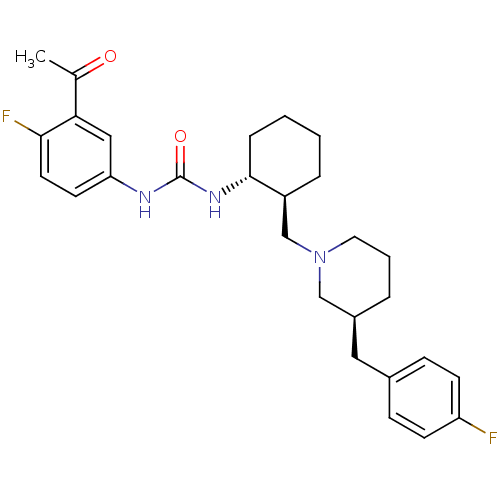
(1-(3-Acetyl-4-fluoro-phenyl)-3-{(1R,2S)-2-[(S)-3-(...)Show SMILES CC(=O)c1cc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)ccc1F Show InChI InChI=1S/C28H35F2N3O2/c1-19(34)25-16-24(12-13-26(25)30)31-28(35)32-27-7-3-2-6-22(27)18-33-14-4-5-21(17-33)15-20-8-10-23(29)11-9-20/h8-13,16,21-22,27H,2-7,14-15,17-18H2,1H3,(H2,31,32,35)/t21-,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410314
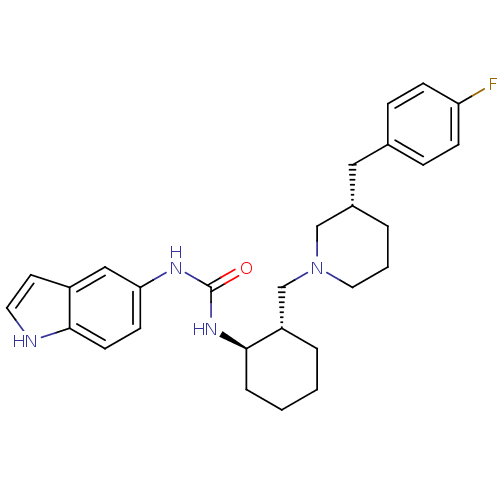
(CHEMBL2113074)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]ccc4c3)C2)cc1 Show InChI InChI=1S/C28H35FN4O/c29-24-9-7-20(8-10-24)16-21-4-3-15-33(18-21)19-23-5-1-2-6-27(23)32-28(34)31-25-11-12-26-22(17-25)13-14-30-26/h7-14,17,21,23,27,30H,1-6,15-16,18-19H2,(H2,31,32,34)/t21-,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of Cynomolgus monkey eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163678

(1-[3,5-Bis-(1-methyl-1H-tetrazol-5-yl)-phenyl]-3-{...)Show SMILES Cn1nnnc1-c1cc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc(c1)-c1nnnn1C Show InChI InChI=1S/C30H38FN11O/c1-40-28(34-36-38-40)23-15-24(29-35-37-39-41(29)2)17-26(16-23)32-30(43)33-27-8-4-3-7-22(27)19-42-13-5-6-21(18-42)14-20-9-11-25(31)12-10-20/h9-12,15-17,21-22,27H,3-8,13-14,18-19H2,1-2H3,(H2,32,33,43)/t21-,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410322
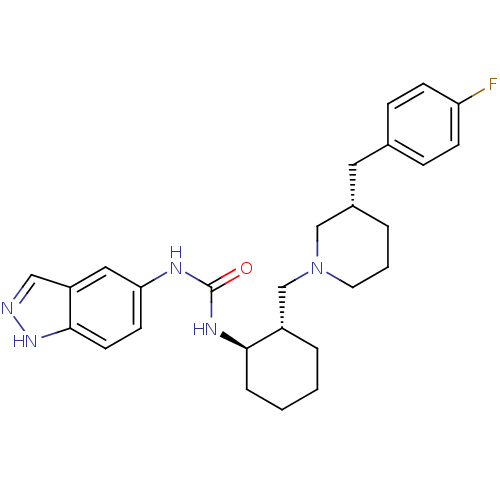
(CHEMBL2113077)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]ncc4c3)C2)cc1 Show InChI InChI=1S/C27H34FN5O/c28-23-9-7-19(8-10-23)14-20-4-3-13-33(17-20)18-21-5-1-2-6-25(21)31-27(34)30-24-11-12-26-22(15-24)16-29-32-26/h7-12,15-16,20-21,25H,1-6,13-14,17-18H2,(H,29,32)(H2,30,31,34)/t20-,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163662
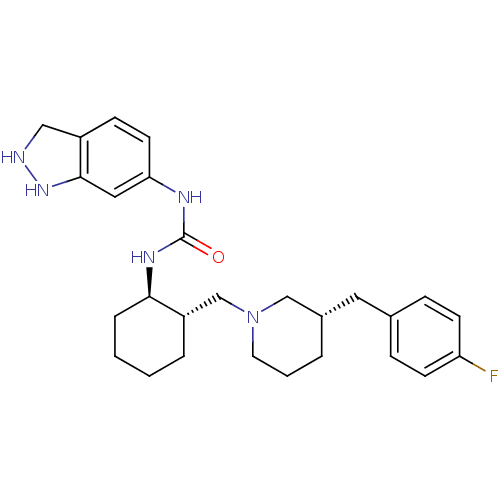
(1-(3a,7a-Dihydro-1H-indazol-6-yl)-3-{(1R,2S)-2-[(S...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4CNNc4c3)C2)cc1 Show InChI InChI=1S/C27H36FN5O/c28-23-10-7-19(8-11-23)14-20-4-3-13-33(17-20)18-22-5-1-2-6-25(22)31-27(34)30-24-12-9-21-16-29-32-26(21)15-24/h7-12,15,20,22,25,29,32H,1-6,13-14,16-18H2,(H2,30,31,34)/t20-,22-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163660
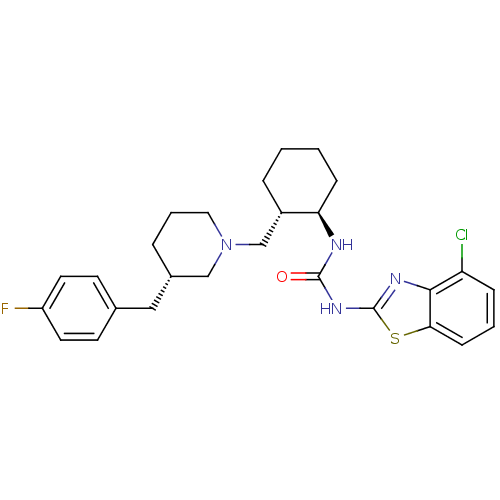
(1-(4-Chloro-benzothiazol-2-yl)-3-{(1R,2S)-2-[(S)-3...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3nc4c(Cl)cccc4s3)C2)cc1 Show InChI InChI=1S/C27H32ClFN4OS/c28-22-7-3-9-24-25(22)31-27(35-24)32-26(34)30-23-8-2-1-6-20(23)17-33-14-4-5-19(16-33)15-18-10-12-21(29)13-11-18/h3,7,9-13,19-20,23H,1-2,4-6,8,14-17H2,(H2,30,31,32,34)/t19-,20-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163656
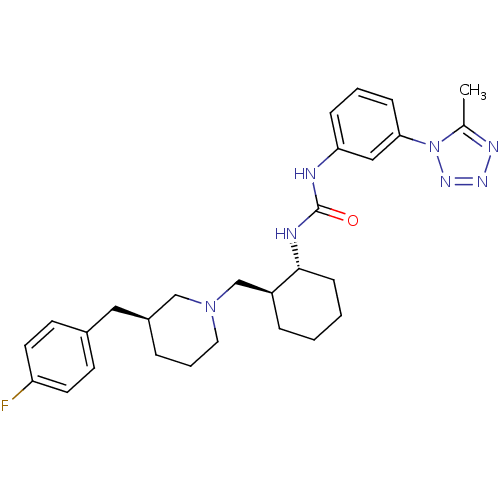
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cc1nnnn1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN7O/c1-20-32-33-34-36(20)26-9-4-8-25(17-26)30-28(37)31-27-10-3-2-7-23(27)19-35-15-5-6-22(18-35)16-21-11-13-24(29)14-12-21/h4,8-9,11-14,17,22-23,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t22-,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163635
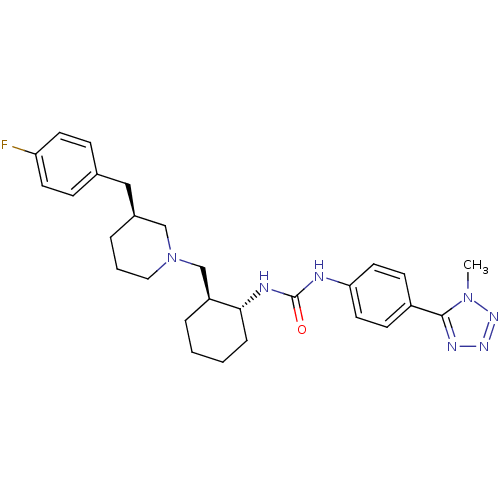
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cn1nnnc1-c1ccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc1 Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-10-14-25(15-11-22)30-28(37)31-26-7-3-2-6-23(26)19-36-16-4-5-21(18-36)17-20-8-12-24(29)13-9-20/h8-15,21,23,26H,2-7,16-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410322

(CHEMBL2113077)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]ncc4c3)C2)cc1 Show InChI InChI=1S/C27H34FN5O/c28-23-9-7-19(8-10-23)14-20-4-3-13-33(17-20)18-21-5-1-2-6-25(21)31-27(34)30-24-11-12-26-22(15-24)16-29-32-26/h7-12,15-16,20-21,25H,1-6,13-14,17-18H2,(H,29,32)(H2,30,31,34)/t20-,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163645
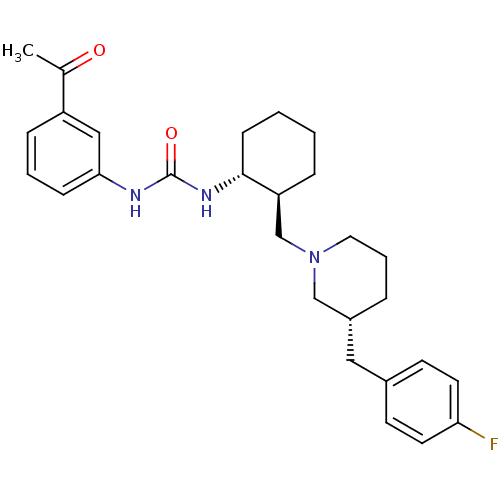
(1-(3-Acetyl-phenyl)-3-{(1R,2S)-2-[(R)-3-(4-fluoro-...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.364 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410314

(CHEMBL2113074)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]ccc4c3)C2)cc1 Show InChI InChI=1S/C28H35FN4O/c29-24-9-7-20(8-10-24)16-21-4-3-15-33(18-21)19-23-5-1-2-6-27(23)32-28(34)31-25-11-12-26-22(17-25)13-14-30-26/h7-14,17,21,23,27,30H,1-6,15-16,18-19H2,(H2,31,32,34)/t21-,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-eotaxin binding to human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-eotaxin binding to human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163635

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cn1nnnc1-c1ccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc1 Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-10-14-25(15-11-22)30-28(37)31-26-7-3-2-6-23(26)19-36-16-4-5-21(18-36)17-20-8-12-24(29)13-9-20/h8-15,21,23,26H,2-7,16-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163662

(1-(3a,7a-Dihydro-1H-indazol-6-yl)-3-{(1R,2S)-2-[(S...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4CNNc4c3)C2)cc1 Show InChI InChI=1S/C27H36FN5O/c28-23-10-7-19(8-11-23)14-20-4-3-13-33(17-20)18-22-5-1-2-6-25(22)31-27(34)30-24-12-9-21-16-29-32-26(21)15-24/h7-12,15,20,22,25,29,32H,1-6,13-14,16-18H2,(H2,30,31,34)/t20-,22-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-eotaxin binding to human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163652
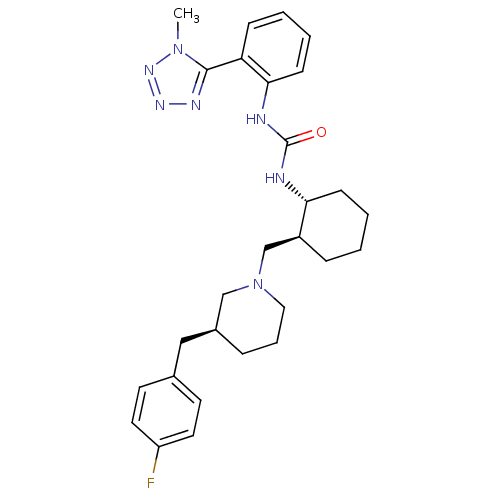
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cn1nnnc1-c1ccccc1NC(=O)N[C@@H]1CCCC[C@H]1CN1CCC[C@@H](Cc2ccc(F)cc2)C1 Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)24-9-3-5-11-26(24)31-28(37)30-25-10-4-2-8-22(25)19-36-16-6-7-21(18-36)17-20-12-14-23(29)15-13-20/h3,5,9,11-15,21-22,25H,2,4,6-8,10,16-19H2,1H3,(H2,30,31,37)/t21-,22-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163632

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES COc1ccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc1 Show InChI InChI=1S/C27H36FN3O2/c1-33-25-14-12-24(13-15-25)29-27(32)30-26-7-3-2-6-22(26)19-31-16-4-5-21(18-31)17-20-8-10-23(28)11-9-20/h8-15,21-22,26H,2-7,16-19H2,1H3,(H2,29,30,32)/t21-,22-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163678

(1-[3,5-Bis-(1-methyl-1H-tetrazol-5-yl)-phenyl]-3-{...)Show SMILES Cn1nnnc1-c1cc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc(c1)-c1nnnn1C Show InChI InChI=1S/C30H38FN11O/c1-40-28(34-36-38-40)23-15-24(29-35-37-39-41(29)2)17-26(16-23)32-30(43)33-27-8-4-3-7-22(27)19-42-13-5-6-21(18-42)14-20-9-11-25(31)12-10-20/h9-12,15-17,21-22,27H,3-8,13-14,18-19H2,1-2H3,(H2,32,33,43)/t21-,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50117461
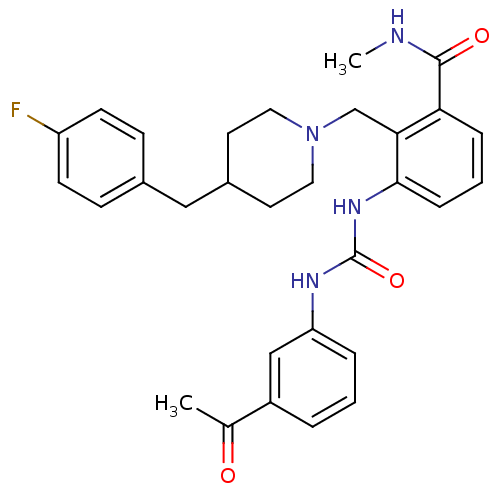
(3-[3-(3-Acetyl-phenyl)-ureido]-2-[4-(4-fluoro-benz...)Show SMILES CNC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(C)=O)c1CN1CCC(Cc2ccc(F)cc2)CC1 Show InChI InChI=1S/C30H33FN4O3/c1-20(36)23-5-3-6-25(18-23)33-30(38)34-28-8-4-7-26(29(37)32-2)27(28)19-35-15-13-22(14-16-35)17-21-9-11-24(31)12-10-21/h3-12,18,22H,13-17,19H2,1-2H3,(H,32,37)(H2,33,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163677
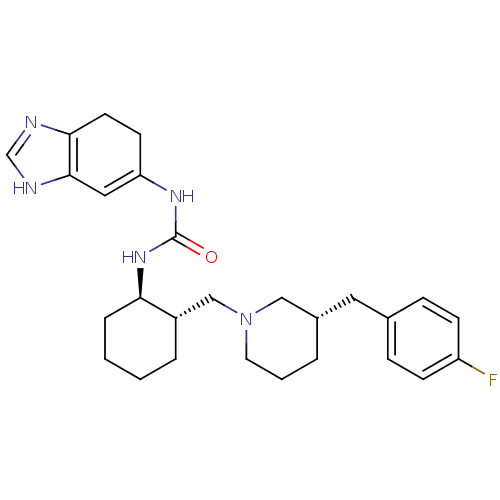
(1-(3a,4-Dihydro-1H-benzoimidazol-5-yl)-3-{(1R,2S)-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)NC3=Cc4[nH]cnc4CC3)C2)cc1 |t:23| Show InChI InChI=1S/C27H36FN5O/c28-22-9-7-19(8-10-22)14-20-4-3-13-33(16-20)17-21-5-1-2-6-24(21)32-27(34)31-23-11-12-25-26(15-23)30-18-29-25/h7-10,15,18,20-21,24H,1-6,11-14,16-17H2,(H,29,30)(H2,31,32,34)/t20-,21-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163682
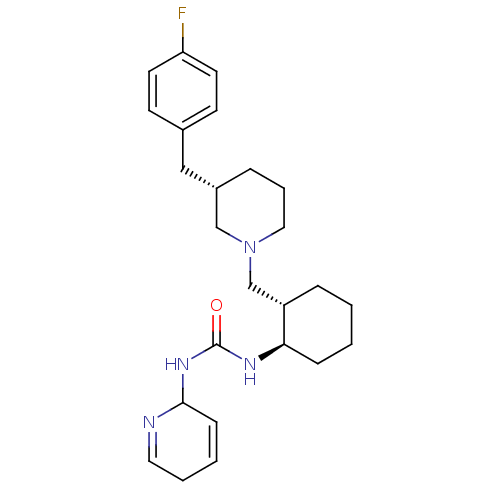
(1-(1,2-Dihydro-pyridin-2-yl)-3-{(1R,2S)-2-[(S)-3-(...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)NC3C=CCC=N3)C2)cc1 |c:24,27| Show InChI InChI=1S/C25H35FN4O/c26-22-12-10-19(11-13-22)16-20-6-5-15-30(17-20)18-21-7-1-2-8-23(21)28-25(31)29-24-9-3-4-14-27-24/h3,9-14,20-21,23-24H,1-2,4-8,15-18H2,(H2,28,29,31)/t20-,21-,23+,24?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163650

(1-(3,5-Diacetyl-phenyl)-3-{(1R,2S)-2-[(S)-3-(4-flu...)Show SMILES CC(=O)c1cc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc(c1)C(C)=O Show InChI InChI=1S/C30H38FN3O3/c1-20(35)25-15-26(21(2)36)17-28(16-25)32-30(37)33-29-8-4-3-7-24(29)19-34-13-5-6-23(18-34)14-22-9-11-27(31)12-10-22/h9-12,15-17,23-24,29H,3-8,13-14,18-19H2,1-2H3,(H2,32,33,37)/t23-,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410321
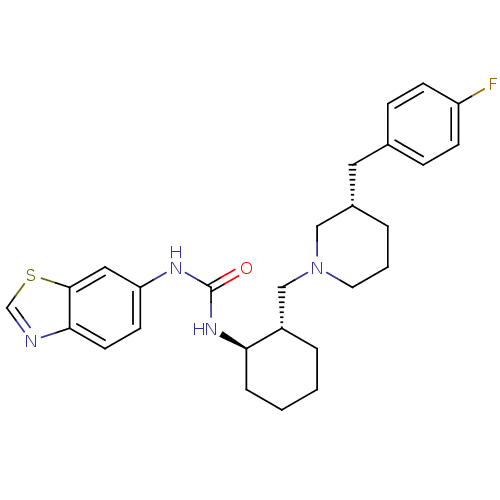
(CHEMBL2113076)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4ncsc4c3)C2)cc1 Show InChI InChI=1S/C27H33FN4OS/c28-22-9-7-19(8-10-22)14-20-4-3-13-32(16-20)17-21-5-1-2-6-24(21)31-27(33)30-23-11-12-25-26(15-23)34-18-29-25/h7-12,15,18,20-21,24H,1-6,13-14,16-17H2,(H2,30,31,33)/t20-,21-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410323
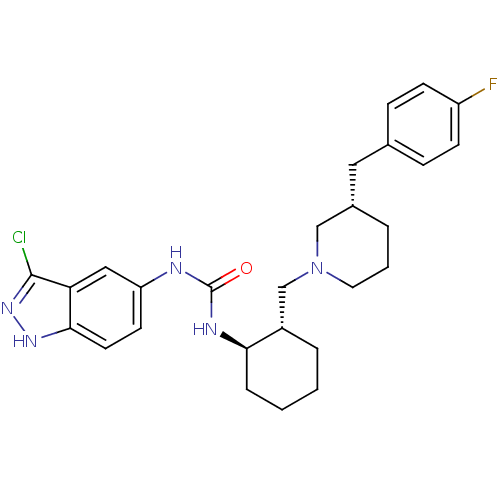
(CHEMBL2113079)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]nc(Cl)c4c3)C2)cc1 Show InChI InChI=1S/C27H33ClFN5O/c28-26-23-15-22(11-12-25(23)32-33-26)30-27(35)31-24-6-2-1-5-20(24)17-34-13-3-4-19(16-34)14-18-7-9-21(29)10-8-18/h7-12,15,19-20,24H,1-6,13-14,16-17H2,(H,32,33)(H2,30,31,35)/t19-,20-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163651
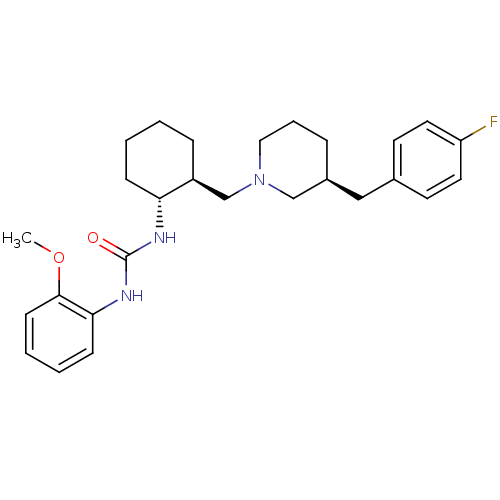
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES COc1ccccc1NC(=O)N[C@@H]1CCCC[C@H]1CN1CCC[C@@H](Cc2ccc(F)cc2)C1 Show InChI InChI=1S/C27H36FN3O2/c1-33-26-11-5-4-10-25(26)30-27(32)29-24-9-3-2-8-22(24)19-31-16-6-7-21(18-31)17-20-12-14-23(28)15-13-20/h4-5,10-15,21-22,24H,2-3,6-9,16-19H2,1H3,(H2,29,30,32)/t21-,22-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163670
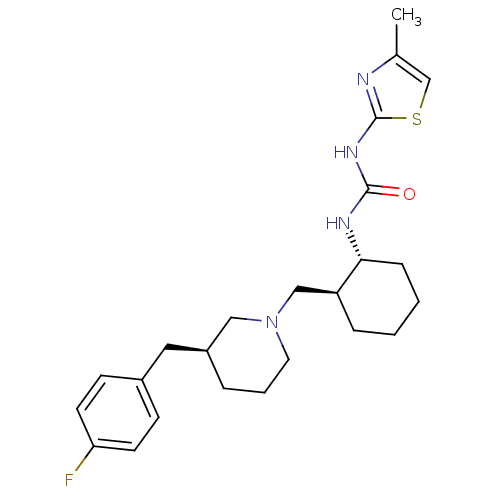
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cc1csc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)n1 Show InChI InChI=1S/C24H33FN4OS/c1-17-16-31-24(26-17)28-23(30)27-22-7-3-2-6-20(22)15-29-12-4-5-19(14-29)13-18-8-10-21(25)11-9-18/h8-11,16,19-20,22H,2-7,12-15H2,1H3,(H2,26,27,28,30)/t19-,20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163656

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cc1nnnn1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN7O/c1-20-32-33-34-36(20)26-9-4-8-25(17-26)30-28(37)31-27-10-3-2-7-23(27)19-35-15-5-6-22(18-35)16-21-11-13-24(29)14-12-21/h4,8-9,11-14,17,22-23,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t22-,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163661

(1-(3-Acetyl-4-fluoro-phenyl)-3-{(1R,2S)-2-[(S)-3-(...)Show SMILES CC(=O)c1cc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)ccc1F Show InChI InChI=1S/C28H35F2N3O2/c1-19(34)25-16-24(12-13-26(25)30)31-28(35)32-27-7-3-2-6-22(27)18-33-14-4-5-21(17-33)15-20-8-10-23(29)11-9-20/h8-13,16,21-22,27H,2-7,14-15,17-18H2,1H3,(H2,31,32,35)/t21-,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163666
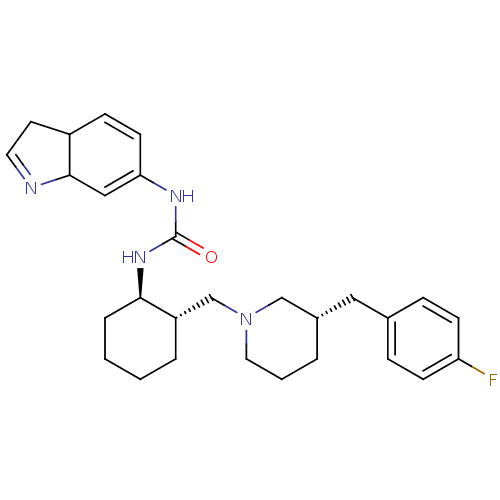
(1-(3a,7a-Dihydro-1H-indol-6-yl)-3-{(1R,2S)-2-[(S)-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)NC3=CC4N=CCC4C=C3)C2)cc1 |c:26,31,t:23| Show InChI InChI=1S/C28H37FN4O/c29-24-10-7-20(8-11-24)16-21-4-3-15-33(18-21)19-23-5-1-2-6-26(23)32-28(34)31-25-12-9-22-13-14-30-27(22)17-25/h7-12,14,17,21-23,26-27H,1-6,13,15-16,18-19H2,(H2,31,32,34)/t21-,22?,23-,26+,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163671
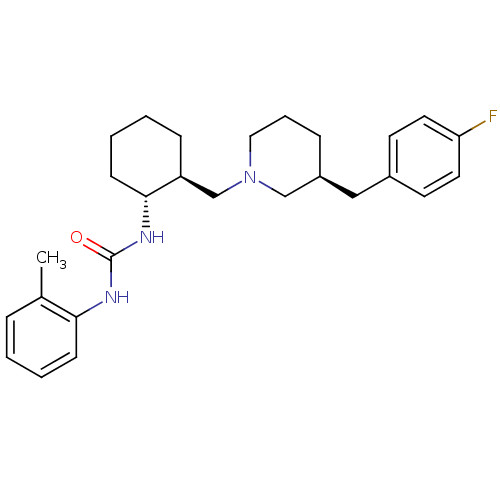
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cc1ccccc1NC(=O)N[C@@H]1CCCC[C@H]1CN1CCC[C@@H](Cc2ccc(F)cc2)C1 Show InChI InChI=1S/C27H36FN3O/c1-20-7-2-4-10-25(20)29-27(32)30-26-11-5-3-9-23(26)19-31-16-6-8-22(18-31)17-21-12-14-24(28)15-13-21/h2,4,7,10,12-15,22-23,26H,3,5-6,8-9,11,16-19H2,1H3,(H2,29,30,32)/t22-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410325
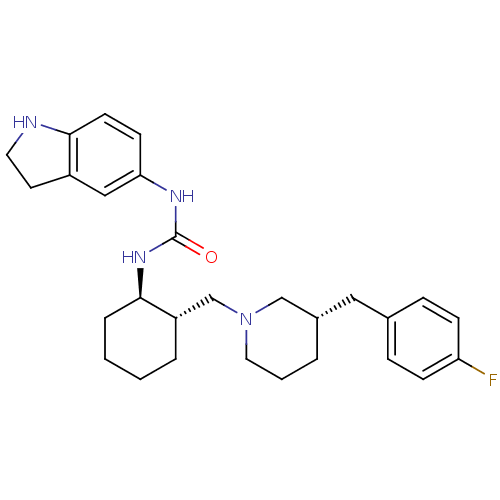
(CHEMBL2113080)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4NCCc4c3)C2)cc1 Show InChI InChI=1S/C28H37FN4O/c29-24-9-7-20(8-10-24)16-21-4-3-15-33(18-21)19-23-5-1-2-6-27(23)32-28(34)31-25-11-12-26-22(17-25)13-14-30-26/h7-12,17,21,23,27,30H,1-6,13-16,18-19H2,(H2,31,32,34)/t21-,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163675
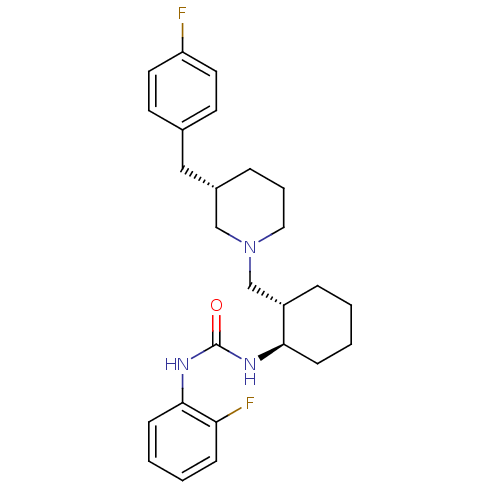
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccccc3F)C2)cc1 Show InChI InChI=1S/C26H33F2N3O/c27-22-13-11-19(12-14-22)16-20-6-5-15-31(17-20)18-21-7-1-3-9-24(21)29-26(32)30-25-10-4-2-8-23(25)28/h2,4,8,10-14,20-21,24H,1,3,5-7,9,15-18H2,(H2,29,30,32)/t20-,21-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163647
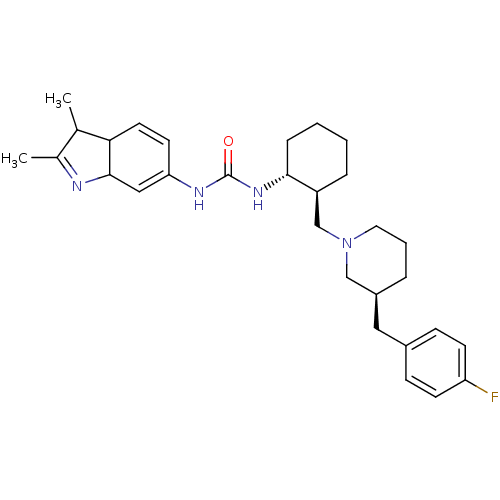
(1-(2,3-Dimethyl-3a,7a-dihydro-1H-indol-6-yl)-3-{(1...)Show SMILES CC1C2C=CC(NC(=O)N[C@@H]3CCCC[C@H]3CN3CCC[C@@H](Cc4ccc(F)cc4)C3)=CC2N=C1C |c:3,33,37| Show InChI InChI=1S/C30H41FN4O/c1-20-21(2)32-29-17-26(13-14-27(20)29)33-30(36)34-28-8-4-3-7-24(28)19-35-15-5-6-23(18-35)16-22-9-11-25(31)12-10-22/h9-14,17,20,23-24,27-29H,3-8,15-16,18-19H2,1-2H3,(H2,33,34,36)/t20?,23-,24-,27?,28+,29?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163637
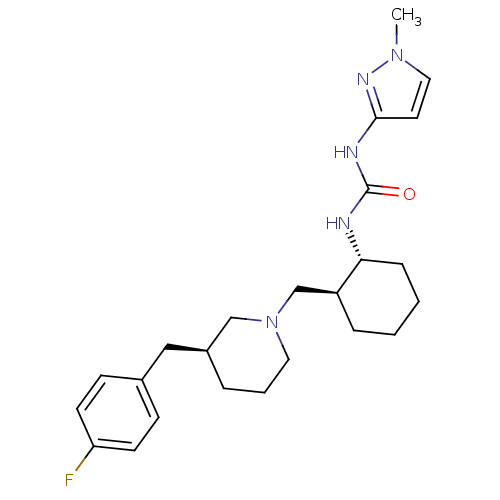
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cn1ccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)n1 Show InChI InChI=1S/C24H34FN5O/c1-29-14-12-23(28-29)27-24(31)26-22-7-3-2-6-20(22)17-30-13-4-5-19(16-30)15-18-8-10-21(25)11-9-18/h8-12,14,19-20,22H,2-7,13,15-17H2,1H3,(H2,26,27,28,31)/t19-,20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163676
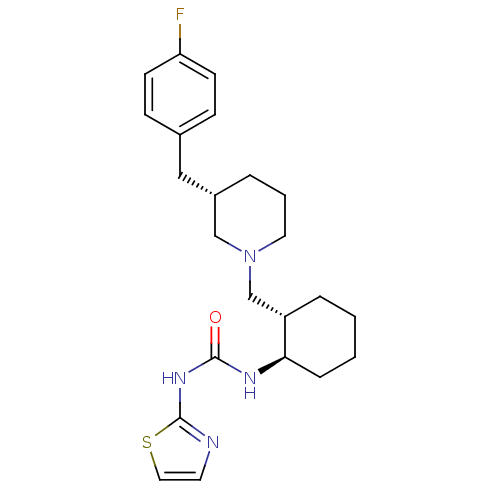
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3nccs3)C2)cc1 Show InChI InChI=1S/C23H31FN4OS/c24-20-9-7-17(8-10-20)14-18-4-3-12-28(15-18)16-19-5-1-2-6-21(19)26-22(29)27-23-25-11-13-30-23/h7-11,13,18-19,21H,1-6,12,14-16H2,(H2,25,26,27,29)/t18-,19-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163641
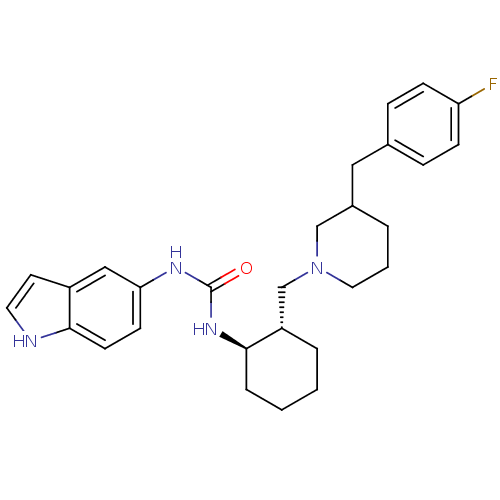
(1-{(1R,2S)-2-[3-(4-Fluoro-benzyl)-piperidin-1-ylme...)Show SMILES Fc1ccc(CC2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]ccc4c3)C2)cc1 Show InChI InChI=1S/C28H35FN4O/c29-24-9-7-20(8-10-24)16-21-4-3-15-33(18-21)19-23-5-1-2-6-27(23)32-28(34)31-25-11-12-26-22(17-25)13-14-30-26/h7-14,17,21,23,27,30H,1-6,15-16,18-19H2,(H2,31,32,34)/t21?,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163644
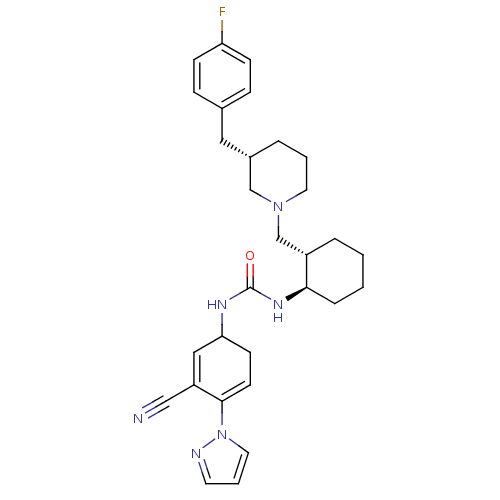
(1-(3-Cyano-4-pyrazol-1-yl-cyclohexa-2,4-dienyl)-3-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)NC3CC=C(C(=C3)C#N)n3cccn3)C2)cc1 |c:25,27| Show InChI InChI=1S/C30H37FN6O/c31-26-10-8-22(9-11-26)17-23-5-3-15-36(20-23)21-24-6-1-2-7-28(24)35-30(38)34-27-12-13-29(25(18-27)19-32)37-16-4-14-33-37/h4,8-11,13-14,16,18,23-24,27-28H,1-3,5-7,12,15,17,20-21H2,(H2,34,35,38)/t23-,24-,27?,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163632

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES COc1ccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc1 Show InChI InChI=1S/C27H36FN3O2/c1-33-25-14-12-24(13-15-25)29-27(32)30-26-7-3-2-6-22(26)19-31-16-4-5-21(18-31)17-20-8-10-23(28)11-9-20/h8-15,21-22,26H,2-7,16-19H2,1H3,(H2,29,30,32)/t21-,22-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163678

(1-[3,5-Bis-(1-methyl-1H-tetrazol-5-yl)-phenyl]-3-{...)Show SMILES Cn1nnnc1-c1cc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc(c1)-c1nnnn1C Show InChI InChI=1S/C30H38FN11O/c1-40-28(34-36-38-40)23-15-24(29-35-37-39-41(29)2)17-26(16-23)32-30(43)33-27-8-4-3-7-22(27)19-42-13-5-6-21(18-42)14-20-9-11-25(31)12-10-20/h9-12,15-17,21-22,27H,3-8,13-14,18-19H2,1-2H3,(H2,32,33,43)/t21-,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410314

(CHEMBL2113074)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]ccc4c3)C2)cc1 Show InChI InChI=1S/C28H35FN4O/c29-24-9-7-20(8-10-24)16-21-4-3-15-33(18-21)19-23-5-1-2-6-27(23)32-28(34)31-25-11-12-26-22(17-25)13-14-30-26/h7-14,17,21,23,27,30H,1-6,15-16,18-19H2,(H2,31,32,34)/t21-,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410322

(CHEMBL2113077)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]ncc4c3)C2)cc1 Show InChI InChI=1S/C27H34FN5O/c28-23-9-7-19(8-10-23)14-20-4-3-13-33(17-20)18-21-5-1-2-6-25(21)31-27(34)30-24-11-12-26-22(15-24)16-29-32-26/h7-12,15-16,20-21,25H,1-6,13-14,17-18H2,(H,29,32)(H2,30,31,34)/t20-,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163650

(1-(3,5-Diacetyl-phenyl)-3-{(1R,2S)-2-[(S)-3-(4-flu...)Show SMILES CC(=O)c1cc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc(c1)C(C)=O Show InChI InChI=1S/C30H38FN3O3/c1-20(35)25-15-26(21(2)36)17-28(16-25)32-30(37)33-29-8-4-3-7-24(29)19-34-13-5-6-23(18-34)14-22-9-11-27(31)12-10-22/h9-12,15-17,23-24,29H,3-8,13-14,18-19H2,1-2H3,(H2,32,33,37)/t23-,24-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163656

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cc1nnnn1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN7O/c1-20-32-33-34-36(20)26-9-4-8-25(17-26)30-28(37)31-27-10-3-2-7-23(27)19-35-15-5-6-22(18-35)16-21-11-13-24(29)14-12-21/h4,8-9,11-14,17,22-23,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t22-,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163641

(1-{(1R,2S)-2-[3-(4-Fluoro-benzyl)-piperidin-1-ylme...)Show SMILES Fc1ccc(CC2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]ccc4c3)C2)cc1 Show InChI InChI=1S/C28H35FN4O/c29-24-9-7-20(8-10-24)16-21-4-3-15-33(18-21)19-23-5-1-2-6-27(23)32-28(34)31-25-11-12-26-22(17-25)13-14-30-26/h7-14,17,21,23,27,30H,1-6,15-16,18-19H2,(H2,31,32,34)/t21?,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163681
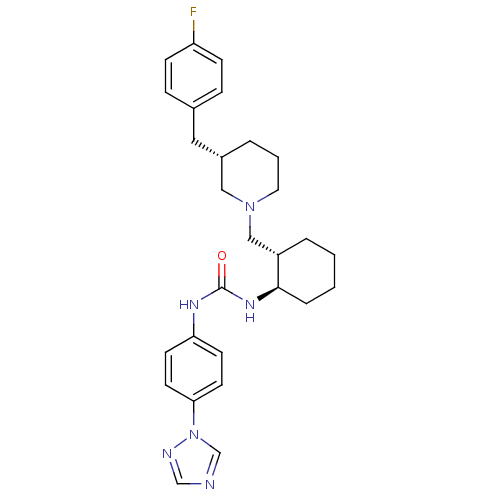
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc(cc3)-n3cncn3)C2)cc1 Show InChI InChI=1S/C28H35FN6O/c29-24-9-7-21(8-10-24)16-22-4-3-15-34(17-22)18-23-5-1-2-6-27(23)33-28(36)32-25-11-13-26(14-12-25)35-20-30-19-31-35/h7-14,19-20,22-23,27H,1-6,15-18H2,(H2,32,33,36)/t22-,23-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163683

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3nncs3)C2)cc1 Show InChI InChI=1S/C22H30FN5OS/c23-19-9-7-16(8-10-19)12-17-4-3-11-28(13-17)14-18-5-1-2-6-20(18)25-21(29)26-22-27-24-15-30-22/h7-10,15,17-18,20H,1-6,11-14H2,(H2,25,26,27,29)/t17-,18-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163659

(1-{(1R,2S)-2-[3-(4-Fluoro-benzyl)-piperidin-1-ylme...)Show SMILES Fc1ccc(CC2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]ncc4c3)C2)cc1 Show InChI InChI=1S/C27H34FN5O/c28-23-9-7-19(8-10-23)14-20-4-3-13-33(17-20)18-21-5-1-2-6-25(21)31-27(34)30-24-11-12-26-22(15-24)16-29-32-26/h7-12,15-16,20-21,25H,1-6,13-14,17-18H2,(H,29,32)(H2,30,31,34)/t20?,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163667
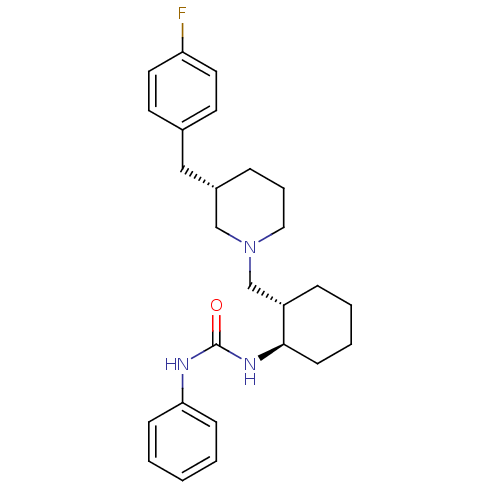
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccccc3)C2)cc1 Show InChI InChI=1S/C26H34FN3O/c27-23-14-12-20(13-15-23)17-21-7-6-16-30(18-21)19-22-8-4-5-11-25(22)29-26(31)28-24-9-2-1-3-10-24/h1-3,9-10,12-15,21-22,25H,4-8,11,16-19H2,(H2,28,29,31)/t21-,22-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163645

(1-(3-Acetyl-phenyl)-3-{(1R,2S)-2-[(R)-3-(4-fluoro-...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50142448

((S)-1-(3-(3-(4-fluorobenzyl)piperidin-1-yl)propyl)...)Show SMILES CC(=O)c1cccc(NC(=O)NCCCN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C24H30FN3O2/c1-18(29)21-6-2-7-23(16-21)27-24(30)26-12-4-14-28-13-3-5-20(17-28)15-19-8-10-22(25)11-9-19/h2,6-11,16,20H,3-5,12-15,17H2,1H3,(H2,26,27,30)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50142448

((S)-1-(3-(3-(4-fluorobenzyl)piperidin-1-yl)propyl)...)Show SMILES CC(=O)c1cccc(NC(=O)NCCCN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C24H30FN3O2/c1-18(29)21-6-2-7-23(16-21)27-24(30)26-12-4-14-28-13-3-5-20(17-28)15-19-8-10-22(25)11-9-19/h2,6-11,16,20H,3-5,12-15,17H2,1H3,(H2,26,27,30)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163645

(1-(3-Acetyl-phenyl)-3-{(1R,2S)-2-[(R)-3-(4-fluoro-...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163638
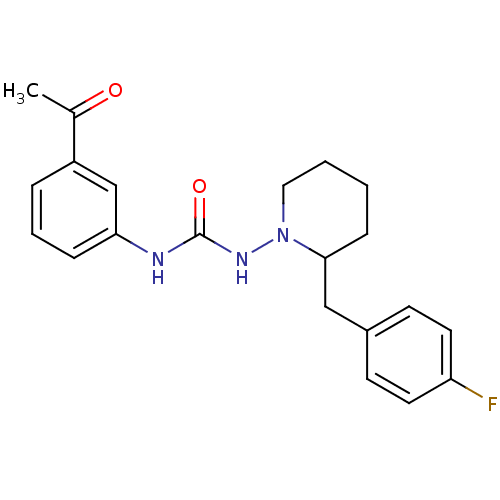
(1-(3-Acetyl-phenyl)-3-[2-(4-fluoro-benzyl)-piperid...)Show SMILES CC(=O)c1cccc(NC(=O)NN2CCCCC2Cc2ccc(F)cc2)c1 Show InChI InChI=1S/C21H24FN3O2/c1-15(26)17-5-4-6-19(14-17)23-21(27)24-25-12-3-2-7-20(25)13-16-8-10-18(22)11-9-16/h4-6,8-11,14,20H,2-3,7,12-13H2,1H3,(H2,23,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163674
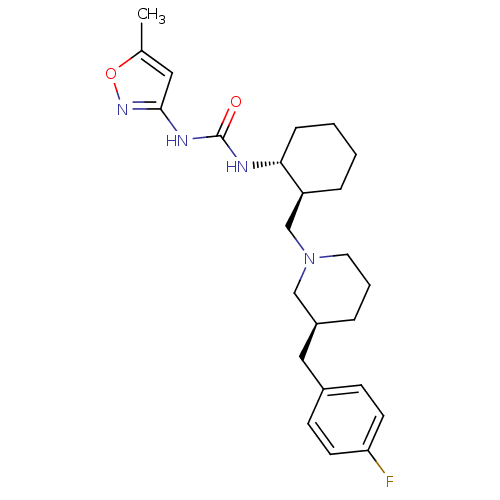
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cc1cc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)no1 Show InChI InChI=1S/C24H33FN4O2/c1-17-13-23(28-31-17)27-24(30)26-22-7-3-2-6-20(22)16-29-12-4-5-19(15-29)14-18-8-10-21(25)11-9-18/h8-11,13,19-20,22H,2-7,12,14-16H2,1H3,(H2,26,27,28,30)/t19-,20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163639

(1-(3-Acetyl-phenyl)-3-{(1R,2S)-2-[4-(4-fluoro-benz...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC(Cc3ccc(F)cc3)CC2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-6-4-7-26(18-23)30-28(34)31-27-8-3-2-5-24(27)19-32-15-13-22(14-16-32)17-21-9-11-25(29)12-10-21/h4,6-7,9-12,18,22,24,27H,2-3,5,8,13-17,19H2,1H3,(H2,30,31,34)/t24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163657

(1-(3-Acetyl-phenyl)-3-[(1R,2S)-2-(4-benzyl-piperid...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC(Cc3ccccc3)CC2)c1 Show InChI InChI=1S/C28H37N3O2/c1-21(32)24-11-7-12-26(19-24)29-28(33)30-27-13-6-5-10-25(27)20-31-16-14-23(15-17-31)18-22-8-3-2-4-9-22/h2-4,7-9,11-12,19,23,25,27H,5-6,10,13-18,20H2,1H3,(H2,29,30,33)/t25-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163660

(1-(4-Chloro-benzothiazol-2-yl)-3-{(1R,2S)-2-[(S)-3...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3nc4c(Cl)cccc4s3)C2)cc1 Show InChI InChI=1S/C27H32ClFN4OS/c28-22-7-3-9-24-25(22)31-27(35-24)32-26(34)30-23-8-2-1-6-20(23)17-33-14-4-5-19(16-33)15-18-10-12-21(29)13-11-18/h3,7,9-13,19-20,23H,1-2,4-6,8,14-17H2,(H2,30,31,32,34)/t19-,20-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163644

(1-(3-Cyano-4-pyrazol-1-yl-cyclohexa-2,4-dienyl)-3-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)NC3CC=C(C(=C3)C#N)n3cccn3)C2)cc1 |c:25,27| Show InChI InChI=1S/C30H37FN6O/c31-26-10-8-22(9-11-26)17-23-5-3-15-36(20-23)21-24-6-1-2-7-28(24)35-30(38)34-27-12-13-29(25(18-27)19-32)37-16-4-14-33-37/h4,8-11,13-14,16,18,23-24,27-28H,1-3,5-7,12,15,17,20-21H2,(H2,34,35,38)/t23-,24-,27?,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163640

(1-(3-Acetyl-phenyl)-3-{2-[4-(4-fluoro-benzyl)-pipe...)Show SMILES CC(=O)c1cccc(NC(=O)NC2CCCCC2CN2CCC(Cc3ccc(F)cc3)CC2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-6-4-7-26(18-23)30-28(34)31-27-8-3-2-5-24(27)19-32-15-13-22(14-16-32)17-21-9-11-25(29)12-10-21/h4,6-7,9-12,18,22,24,27H,2-3,5,8,13-17,19H2,1H3,(H2,30,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163661

(1-(3-Acetyl-4-fluoro-phenyl)-3-{(1R,2S)-2-[(S)-3-(...)Show SMILES CC(=O)c1cc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)ccc1F Show InChI InChI=1S/C28H35F2N3O2/c1-19(34)25-16-24(12-13-26(25)30)31-28(35)32-27-7-3-2-6-22(27)18-33-14-4-5-21(17-33)15-20-8-10-23(29)11-9-20/h8-13,16,21-22,27H,2-7,14-15,17-18H2,1H3,(H2,31,32,35)/t21-,22-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163662

(1-(3a,7a-Dihydro-1H-indazol-6-yl)-3-{(1R,2S)-2-[(S...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4CNNc4c3)C2)cc1 Show InChI InChI=1S/C27H36FN5O/c28-23-10-7-19(8-11-23)14-20-4-3-13-33(17-20)18-22-5-1-2-6-25(22)31-27(34)30-24-12-9-21-16-29-32-26(21)15-24/h7-12,15,20,22,25,29,32H,1-6,13-14,16-18H2,(H2,30,31,34)/t20-,22-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163648

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cc1ccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc1F Show InChI InChI=1S/C27H35F2N3O/c1-19-8-13-24(16-25(19)29)30-27(33)31-26-7-3-2-6-22(26)18-32-14-4-5-21(17-32)15-20-9-11-23(28)12-10-20/h8-13,16,21-22,26H,2-7,14-15,17-18H2,1H3,(H2,30,31,33)/t21-,22-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163680

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cc1ccc(F)cc1NC(=O)N[C@@H]1CCCC[C@H]1CN1CCC[C@@H](Cc2ccc(F)cc2)C1 Show InChI InChI=1S/C27H35F2N3O/c1-19-8-11-24(29)16-26(19)31-27(33)30-25-7-3-2-6-22(25)18-32-14-4-5-21(17-32)15-20-9-12-23(28)13-10-20/h8-13,16,21-22,25H,2-7,14-15,17-18H2,1H3,(H2,30,31,33)/t21-,22-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163654
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc(F)cc3)C2)cc1 Show InChI InChI=1S/C26H33F2N3O/c27-22-9-7-19(8-10-22)16-20-4-3-15-31(17-20)18-21-5-1-2-6-25(21)30-26(32)29-24-13-11-23(28)12-14-24/h7-14,20-21,25H,1-6,15-18H2,(H2,29,30,32)/t20-,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163646
(1-(4-Bromo-phenyl)-3-{(1R,2S)-2-[(S)-3-(4-fluoro-b...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc(Br)cc3)C2)cc1 Show InChI InChI=1S/C26H33BrFN3O/c27-22-9-13-24(14-10-22)29-26(32)30-25-6-2-1-5-21(25)18-31-15-3-4-20(17-31)16-19-7-11-23(28)12-8-19/h7-14,20-21,25H,1-6,15-18H2,(H2,29,30,32)/t20-,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410317

(CHEMBL2096696)Show SMILES Fc1ccc(C[C@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3cccc(c3)C#N)C2)cc1 Show InChI InChI=1S/C27H33FN4O/c28-24-12-10-20(11-13-24)15-22-6-4-14-32(18-22)19-23-7-1-2-9-26(23)31-27(33)30-25-8-3-5-21(16-25)17-29/h3,5,8,10-13,16,22-23,26H,1-2,4,6-7,9,14-15,18-19H2,(H2,30,31,33)/t22-,23+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410318
(CHEMBL2096764)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3cccc(c3)C#N)C2)cc1 Show InChI InChI=1S/C27H33FN4O/c28-24-12-10-20(11-13-24)15-22-6-4-14-32(18-22)19-23-7-1-2-9-26(23)31-27(33)30-25-8-3-5-21(16-25)17-29/h3,5,8,10-13,16,22-23,26H,1-2,4,6-7,9,14-15,18-19H2,(H2,30,31,33)/t22-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163664
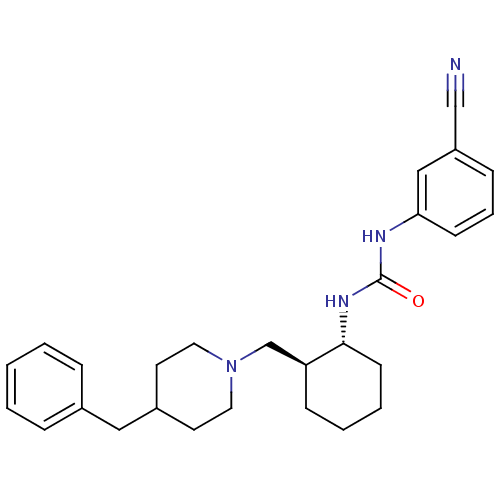
(1-[(1R,2S)-2-(4-Benzyl-piperidin-1-ylmethyl)-cyclo...)Show SMILES O=C(N[C@@H]1CCCC[C@H]1CN1CCC(Cc2ccccc2)CC1)Nc1cccc(c1)C#N Show InChI InChI=1S/C27H34N4O/c28-19-23-9-6-11-25(18-23)29-27(32)30-26-12-5-4-10-24(26)20-31-15-13-22(14-16-31)17-21-7-2-1-3-8-21/h1-3,6-9,11,18,22,24,26H,4-5,10,12-17,20H2,(H2,29,30,32)/t24-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50115023

(1-(3-Acetyl-phenyl)-3-{3-[4-(4-fluoro-benzyl)-pipe...)Show SMILES CC(=O)c1cccc(NC(=O)NCCCN2CCC(Cc3ccc(F)cc3)CC2)c1 Show InChI InChI=1S/C24H30FN3O2/c1-18(29)21-4-2-5-23(17-21)27-24(30)26-12-3-13-28-14-10-20(11-15-28)16-19-6-8-22(25)9-7-19/h2,4-9,17,20H,3,10-16H2,1H3,(H2,26,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163651

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES COc1ccccc1NC(=O)N[C@@H]1CCCC[C@H]1CN1CCC[C@@H](Cc2ccc(F)cc2)C1 Show InChI InChI=1S/C27H36FN3O2/c1-33-26-11-5-4-10-25(26)30-27(32)29-24-9-3-2-8-22(24)19-31-16-6-7-21(18-31)17-20-12-14-23(28)15-13-20/h4-5,10-15,21-22,24H,2-3,6-9,16-19H2,1H3,(H2,29,30,32)/t21-,22-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163665
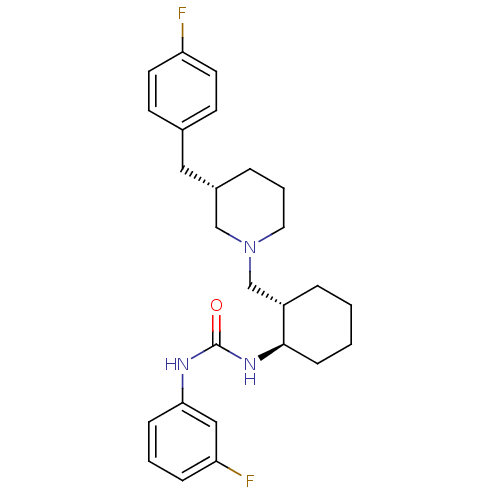
(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3cccc(F)c3)C2)cc1 Show InChI InChI=1S/C26H33F2N3O/c27-22-12-10-19(11-13-22)15-20-5-4-14-31(17-20)18-21-6-1-2-9-25(21)30-26(32)29-24-8-3-7-23(28)16-24/h3,7-8,10-13,16,20-21,25H,1-2,4-6,9,14-15,17-18H2,(H2,29,30,32)/t20-,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
Probable C-C chemokine receptor type 3
(Mus musculus) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of mouse eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163658

(1-(3-Cyano-phenyl)-3-{(1R,2S)-2-[4-(4-fluoro-benzy...)Show SMILES Fc1ccc(CC2CCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3cccc(c3)C#N)CC2)cc1 Show InChI InChI=1S/C27H33FN4O/c28-24-10-8-20(9-11-24)16-21-12-14-32(15-13-21)19-23-5-1-2-7-26(23)31-27(33)30-25-6-3-4-22(17-25)18-29/h3-4,6,8-11,17,21,23,26H,1-2,5,7,12-16,19H2,(H2,30,31,33)/t23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163643

(1-{2-[4-(4-Fluoro-benzyl)-piperidin-1-ylmethyl]-cy...)Show SMILES COc1cccc(NC(=O)NC2CCCCC2CN2CCC(Cc3ccc(F)cc3)CC2)c1 Show InChI InChI=1S/C27H36FN3O2/c1-33-25-7-4-6-24(18-25)29-27(32)30-26-8-3-2-5-22(26)19-31-15-13-21(14-16-31)17-20-9-11-23(28)12-10-20/h4,6-7,9-12,18,21-22,26H,2-3,5,8,13-17,19H2,1H3,(H2,29,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163684
(1-(3,4-Difluoro-phenyl)-3-{(1R,2S)-2-[(S)-3-(4-flu...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc(F)c(F)c3)C2)cc1 Show InChI InChI=1S/C26H32F3N3O/c27-21-9-7-18(8-10-21)14-19-4-3-13-32(16-19)17-20-5-1-2-6-25(20)31-26(33)30-22-11-12-23(28)24(29)15-22/h7-12,15,19-20,25H,1-6,13-14,16-17H2,(H2,30,31,33)/t19-,20-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163679

(1-{(1R,2S)-2-[4-(4-Fluoro-benzyl)-piperidin-1-ylme...)Show SMILES COc1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC(Cc3ccc(F)cc3)CC2)c1 Show InChI InChI=1S/C27H36FN3O2/c1-33-25-7-4-6-24(18-25)29-27(32)30-26-8-3-2-5-22(26)19-31-15-13-21(14-16-31)17-20-9-11-23(28)12-10-20/h4,6-7,9-12,18,21-22,26H,2-3,5,8,13-17,19H2,1H3,(H2,29,30,32)/t22-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410315

(CHEMBL2096763)Show SMILES COc1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C27H36FN3O2/c1-33-25-9-4-8-24(17-25)29-27(32)30-26-10-3-2-7-22(26)19-31-15-5-6-21(18-31)16-20-11-13-23(28)14-12-20/h4,8-9,11-14,17,21-22,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,29,30,32)/t21-,22-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163667

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccccc3)C2)cc1 Show InChI InChI=1S/C26H34FN3O/c27-23-14-12-20(13-15-23)17-21-7-6-16-30(18-21)19-22-8-4-5-11-25(22)29-26(31)28-24-9-2-1-3-10-24/h1-3,9-10,12-15,21-22,25H,4-8,11,16-19H2,(H2,28,29,31)/t21-,22-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
Probable C-C chemokine receptor type 3
(Mus musculus) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of mouse eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163659

(1-{(1R,2S)-2-[3-(4-Fluoro-benzyl)-piperidin-1-ylme...)Show SMILES Fc1ccc(CC2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc4[nH]ncc4c3)C2)cc1 Show InChI InChI=1S/C27H34FN5O/c28-23-9-7-19(8-10-23)14-20-4-3-13-33(17-20)18-21-5-1-2-6-25(21)31-27(34)30-24-11-12-26-22(15-24)16-29-32-26/h7-12,15-16,20-21,25H,1-6,13-14,17-18H2,(H,29,32)(H2,30,31,34)/t20?,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410313
(CHEMBL2112987)Show SMILES COc1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCCC(Cc3ccccc3)C2)c1 Show InChI InChI=1S/C27H37N3O2/c1-32-25-14-7-13-24(18-25)28-27(31)29-26-15-6-5-12-23(26)20-30-16-8-11-22(19-30)17-21-9-3-2-4-10-21/h2-4,7,9-10,13-14,18,22-23,26H,5-6,8,11-12,15-17,19-20H2,1H3,(H2,28,29,31)/t22?,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410324
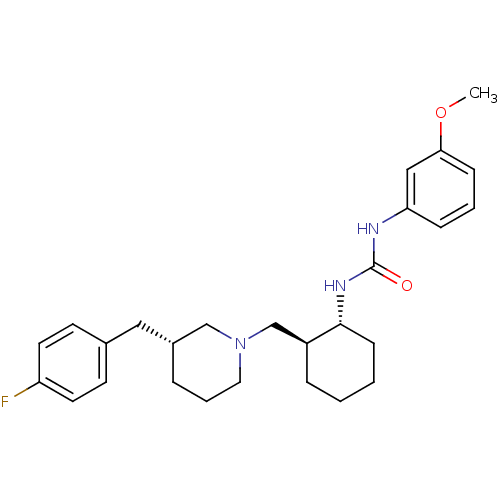
(CHEMBL2096695)Show SMILES COc1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C27H36FN3O2/c1-33-25-9-4-8-24(17-25)29-27(32)30-26-10-3-2-7-22(26)19-31-15-5-6-21(18-31)16-20-11-13-23(28)14-12-20/h4,8-9,11-14,17,21-22,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,29,30,32)/t21-,22+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50117461

(3-[3-(3-Acetyl-phenyl)-ureido]-2-[4-(4-fluoro-benz...)Show SMILES CNC(=O)c1cccc(NC(=O)Nc2cccc(c2)C(C)=O)c1CN1CCC(Cc2ccc(F)cc2)CC1 Show InChI InChI=1S/C30H33FN4O3/c1-20(36)23-5-3-6-25(18-23)33-30(38)34-28-8-4-7-26(29(37)32-2)27(28)19-35-15-13-22(14-16-35)17-21-9-11-24(31)12-10-21/h3-12,18,22H,13-17,19H2,1-2H3,(H,32,37)(H2,33,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410319
(CHEMBL2113082)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3cccc(Cl)c3)C2)cc1 Show InChI InChI=1S/C26H33ClFN3O/c27-22-7-3-8-24(16-22)29-26(32)30-25-9-2-1-6-21(25)18-31-14-4-5-20(17-31)15-19-10-12-23(28)13-11-19/h3,7-8,10-13,16,20-21,25H,1-2,4-6,9,14-15,17-18H2,(H2,29,30,32)/t20-,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163648

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cc1ccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)cc1F Show InChI InChI=1S/C27H35F2N3O/c1-19-8-13-24(16-25(19)29)30-27(33)31-26-7-3-2-6-22(26)18-32-14-4-5-21(17-32)15-20-9-11-23(28)12-10-20/h8-13,16,21-22,26H,2-7,14-15,17-18H2,1H3,(H2,30,31,33)/t21-,22-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
Probable C-C chemokine receptor type 3
(Mus musculus) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of mouse eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410320
(CHEMBL2112049)Show SMILES CC(=O)c1cccc(NC(=O)NC2CCCC(CN3CCC(Cc4ccc(F)cc4)CC3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)24-5-3-7-27(18-24)31-28(34)30-26-6-2-4-23(17-26)19-32-14-12-22(13-15-32)16-21-8-10-25(29)11-9-21/h3,5,7-11,18,22-23,26H,2,4,6,12-17,19H2,1H3,(H2,30,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163646

(1-(4-Bromo-phenyl)-3-{(1R,2S)-2-[(S)-3-(4-fluoro-b...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc(Br)cc3)C2)cc1 Show InChI InChI=1S/C26H33BrFN3O/c27-22-9-13-24(14-10-22)29-26(32)30-25-6-2-1-5-21(25)18-31-15-3-4-20(17-31)16-19-7-11-23(28)12-8-19/h7-14,20-21,25H,1-6,15-18H2,(H2,29,30,32)/t20-,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163654

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Fc1ccc(C[C@@H]2CCCN(C[C@@H]3CCCC[C@H]3NC(=O)Nc3ccc(F)cc3)C2)cc1 Show InChI InChI=1S/C26H33F2N3O/c27-22-9-7-19(8-10-22)16-20-4-3-15-31(17-20)18-21-5-1-2-6-25(21)30-26(32)29-24-13-11-23(28)12-14-24/h7-14,20-21,25H,1-6,15-18H2,(H2,29,30,32)/t20-,21-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of calcium mobilization in human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163638

(1-(3-Acetyl-phenyl)-3-[2-(4-fluoro-benzyl)-piperid...)Show SMILES CC(=O)c1cccc(NC(=O)NN2CCCCC2Cc2ccc(F)cc2)c1 Show InChI InChI=1S/C21H24FN3O2/c1-15(26)17-5-4-6-19(14-17)23-21(27)24-25-12-3-2-7-20(25)13-16-8-10-18(22)11-9-16/h4-6,8-11,14,20H,2-3,7,12-13H2,1H3,(H2,23,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50410316
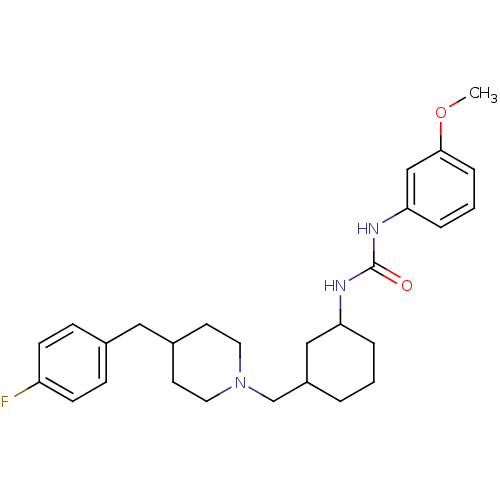
(CHEMBL2112050)Show SMILES COc1cccc(NC(=O)NC2CCCC(CN3CCC(Cc4ccc(F)cc4)CC3)C2)c1 Show InChI InChI=1S/C27H36FN3O2/c1-33-26-7-3-6-25(18-26)30-27(32)29-24-5-2-4-22(17-24)19-31-14-12-21(13-15-31)16-20-8-10-23(28)11-9-20/h3,6-11,18,21-22,24H,2,4-5,12-17,19H2,1H3,(H2,29,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163671

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES Cc1ccccc1NC(=O)N[C@@H]1CCCC[C@H]1CN1CCC[C@@H](Cc2ccc(F)cc2)C1 Show InChI InChI=1S/C27H36FN3O/c1-20-7-2-4-10-25(20)29-27(32)30-26-11-5-3-9-23(26)19-31-16-6-8-22(18-31)17-21-12-14-24(28)15-13-21/h2,4,7,10,12-15,22-23,26H,3,5-6,8-9,11,16-19H2,1H3,(H2,29,30,32)/t22-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163651

(1-{(1R,2S)-2-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-...)Show SMILES COc1ccccc1NC(=O)N[C@@H]1CCCC[C@H]1CN1CCC[C@@H](Cc2ccc(F)cc2)C1 Show InChI InChI=1S/C27H36FN3O2/c1-33-26-11-5-4-10-25(26)30-27(32)29-24-9-3-2-8-22(24)19-31-16-6-7-21(18-31)17-20-12-14-23(28)15-13-20/h4-5,10-15,21-22,24H,2-3,6-9,16-19H2,1H3,(H2,29,30,32)/t21-,22-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50117413

(1-(3-Acetyl-phenyl)-3-{2-[4-(4-fluoro-benzyl)-pipe...)Show SMILES CC(=O)c1cccc(NC(=O)Nc2ccccc2CN2CCC(Cc3ccc(F)cc3)CC2)c1 Show InChI InChI=1S/C28H30FN3O2/c1-20(33)23-6-4-7-26(18-23)30-28(34)31-27-8-3-2-5-24(27)19-32-15-13-22(14-16-32)17-21-9-11-25(29)12-10-21/h2-12,18,22H,13-17,19H2,1H3,(H2,30,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163672

(1-[2-(4-Benzyl-piperidin-1-ylmethyl)-cyclohexyl]-3...)Show SMILES COc1cccc(NC(=O)NC2CCCCC2CN2CCC(Cc3ccccc3)CC2)c1 Show InChI InChI=1S/C27H37N3O2/c1-32-25-12-7-11-24(19-25)28-27(31)29-26-13-6-5-10-23(26)20-30-16-14-22(15-17-30)18-21-8-3-2-4-9-21/h2-4,7-9,11-12,19,22-23,26H,5-6,10,13-18,20H2,1H3,(H2,28,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 423 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of eotaxin-induced chemotaxis of human eosinophils |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50163672

(1-[2-(4-Benzyl-piperidin-1-ylmethyl)-cyclohexyl]-3...)Show SMILES COc1cccc(NC(=O)NC2CCCCC2CN2CCC(Cc3ccccc3)CC2)c1 Show InChI InChI=1S/C27H37N3O2/c1-32-25-12-7-11-24(19-25)28-27(31)29-26-13-6-5-10-23(26)20-30-16-14-22(15-17-30)18-21-8-3-2-4-9-21/h2-4,7-9,11-12,19,22-23,26H,5-6,10,13-18,20H2,1H3,(H2,28,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 423 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cells |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-C chemokine receptor type 5 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-C chemokine receptor type 5 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-C chemokine receptor type 5 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-X-C chemokine receptor type 2 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-X-C chemokine receptor type 2 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-C chemokine receptor type 2 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-C chemokine receptor type 1 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-X-C chemokine receptor type 2 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-C chemokine receptor type 2 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-X-C chemokine receptor type 1 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-X-C chemokine receptor type 1 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50209971

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 Show InChI InChI=1S/C28H36FN3O2/c1-20(33)23-8-4-9-26(17-23)30-28(34)31-27-10-3-2-7-24(27)19-32-15-5-6-22(18-32)16-21-11-13-25(29)14-12-21/h4,8-9,11-14,17,22,24,27H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,34)/t22-,24-,27+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-X-C chemokine receptor type 1 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-C chemokine receptor type 2 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50163634

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES Cn1nnnc1-c1cccc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)c1 |r| Show InChI InChI=1S/C28H36FN7O/c1-35-27(32-33-34-35)22-8-4-9-25(17-22)30-28(37)31-26-10-3-2-7-23(26)19-36-15-5-6-21(18-36)16-20-11-13-24(29)14-12-20/h4,8-9,11-14,17,21,23,26H,2-3,5-7,10,15-16,18-19H2,1H3,(H2,30,31,37)/t21-,23-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-C chemokine receptor type 1 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50163636

(1-((1R,2S)-2-(((S)-3-(4-fluorobenzyl)piperidin-1-y...)Show SMILES CC(=O)c1sc(NC(=O)N[C@@H]2CCCC[C@H]2CN2CCC[C@@H](Cc3ccc(F)cc3)C2)nc1C Show InChI InChI=1S/C26H35FN4O2S/c1-17-24(18(2)32)34-26(28-17)30-25(33)29-23-8-4-3-7-21(23)16-31-13-5-6-20(15-31)14-19-9-11-22(27)12-10-19/h9-12,20-21,23H,3-8,13-16H2,1-2H3,(H2,28,29,30,33)/t20-,21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of C-C chemokine receptor type 1 |
J Med Chem 48: 2194-211 (2005)
Article DOI: 10.1021/jm049530m
BindingDB Entry DOI: 10.7270/Q2XP74F1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data