 Found 28 hits of Enzyme Inhibition Constant Data
Found 28 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50163831
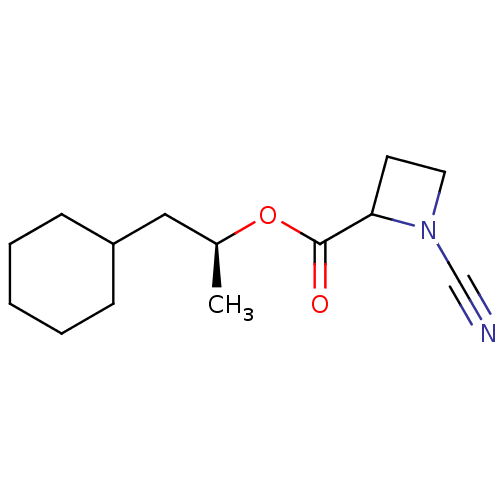
((2S)-1-cyclohexylpropan-2-yl 1-cyanoazetidine-2-ca...)Show InChI InChI=1S/C14H22N2O2/c1-11(9-12-5-3-2-4-6-12)18-14(17)13-7-8-16(13)10-15/h11-13H,2-9H2,1H3/t11-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50148310

(((S)-1-Formyl-pentyl)-carbamic acid (S)-1-benzyl-p...)Show InChI InChI=1S/C17H25NO3/c1-3-5-11-15(13-19)18-17(20)21-16(4-2)12-14-9-7-6-8-10-14/h6-10,13,15-16H,3-5,11-12H2,1-2H3,(H,18,20)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50163831

((2S)-1-cyclohexylpropan-2-yl 1-cyanoazetidine-2-ca...)Show InChI InChI=1S/C14H22N2O2/c1-11(9-12-5-3-2-4-6-12)18-14(17)13-7-8-16(13)10-15/h11-13H,2-9H2,1H3/t11-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin L using 5 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163832
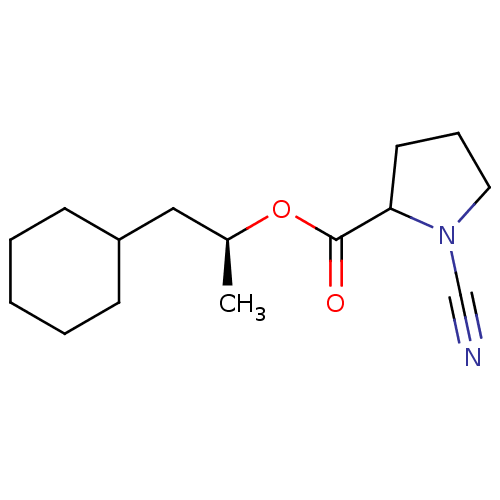
((1S)-2-cyclohexyl-1-methylethyl (2S)-1-cyanopyrrol...)Show InChI InChI=1S/C15H24N2O2/c1-12(10-13-6-3-2-4-7-13)19-15(18)14-8-5-9-17(14)11-16/h12-14H,2-10H2,1H3/t12-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50163831

((2S)-1-cyclohexylpropan-2-yl 1-cyanoazetidine-2-ca...)Show InChI InChI=1S/C14H22N2O2/c1-11(9-12-5-3-2-4-6-12)18-14(17)13-7-8-16(13)10-15/h11-13H,2-9H2,1H3/t11-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin B using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50148291
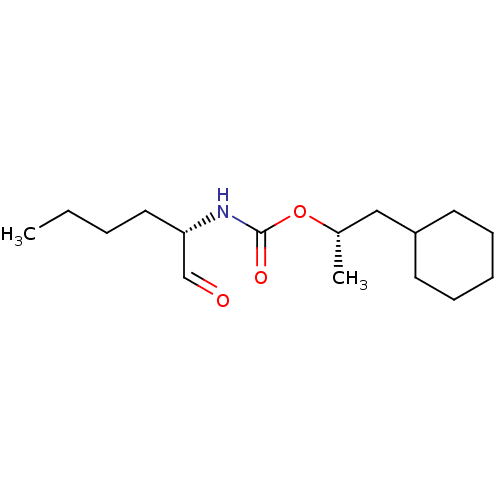
(((S)-1-Formyl-pentyl)-carbamic acid (S)-2-cyclohex...)Show InChI InChI=1S/C16H29NO3/c1-3-4-10-15(12-18)17-16(19)20-13(2)11-14-8-6-5-7-9-14/h12-15H,3-11H2,1-2H3,(H,17,19)/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163841

((1-Cyano-pyrrolidin-3-yl)-carbamic acid (S)-1-cycl...)Show InChI InChI=1S/C16H27N3O2/c1-2-15(10-13-6-4-3-5-7-13)21-16(20)18-14-8-9-19(11-14)12-17/h13-15H,2-11H2,1H3,(H,18,20)/t14?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163838

((1-Cyano-pyrrolidin-3-yl)-carbamic acid (S)-2-cycl...)Show InChI InChI=1S/C15H25N3O2/c1-12(9-13-5-3-2-4-6-13)20-15(19)17-14-7-8-18(10-14)11-16/h12-14H,2-10H2,1H3,(H,17,19)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163842

(CHEMBL183658 | [1-((S)-1-Cyano-pyrrolidin-3-ylcarb...)Show SMILES CC(C)C[C@H](NC(=O)OCC1CCCCC1)C(=O)NC1CCN(C1)C#N Show InChI InChI=1S/C19H32N4O3/c1-14(2)10-17(18(24)21-16-8-9-23(11-16)13-20)22-19(25)26-12-15-6-4-3-5-7-15/h14-17H,3-12H2,1-2H3,(H,21,24)(H,22,25)/t16?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Pro-cathepsin H
(Homo sapiens (Human)) | BDBM50163831

((2S)-1-cyclohexylpropan-2-yl 1-cyanoazetidine-2-ca...)Show InChI InChI=1S/C14H22N2O2/c1-11(9-12-5-3-2-4-6-12)18-14(17)13-7-8-16(13)10-15/h11-13H,2-9H2,1H3/t11-,13?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin H using 50 uM L-Arg-beta-naphthalamide |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163836

(((R)-1-Formyl-pentyl)-carbamic acid tert-butyl est...)Show InChI InChI=1S/C11H21NO3/c1-5-6-7-9(8-13)12-10(14)15-11(2,3)4/h8-9H,5-7H2,1-4H3,(H,12,14)/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163834
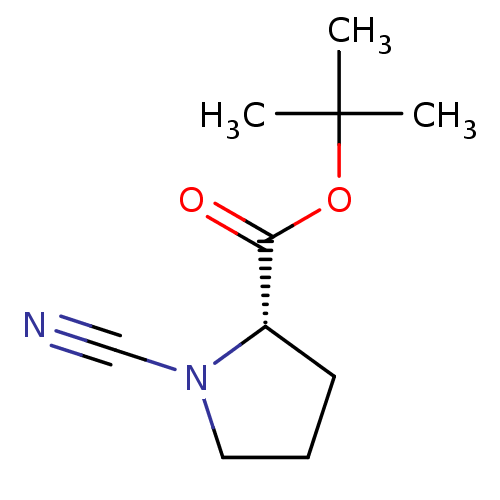
((S)-1-Cyano-pyrrolidine-2-carboxylic acid tert-but...)Show InChI InChI=1S/C10H16N2O2/c1-10(2,3)14-9(13)8-5-4-6-12(8)7-11/h8H,4-6H2,1-3H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50163838

((1-Cyano-pyrrolidin-3-yl)-carbamic acid (S)-2-cycl...)Show InChI InChI=1S/C15H25N3O2/c1-12(9-13-5-3-2-4-6-13)20-15(19)17-14-7-8-18(10-14)11-16/h12-14H,2-10H2,1H3,(H,17,19)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin L using 5 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50024322

(CHEMBL2096777)Show InChI InChI=1S/C10H17N3O2/c1-10(2,3)15-9(14)12-8-4-5-13(6-8)7-11/h8H,4-6H2,1-3H3,(H,12,14)/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50163832

((1S)-2-cyclohexyl-1-methylethyl (2S)-1-cyanopyrrol...)Show InChI InChI=1S/C15H24N2O2/c1-12(10-13-6-3-2-4-7-13)19-15(18)14-8-5-9-17(14)11-16/h12-14H,2-10H2,1H3/t12-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin L using 5 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50024323
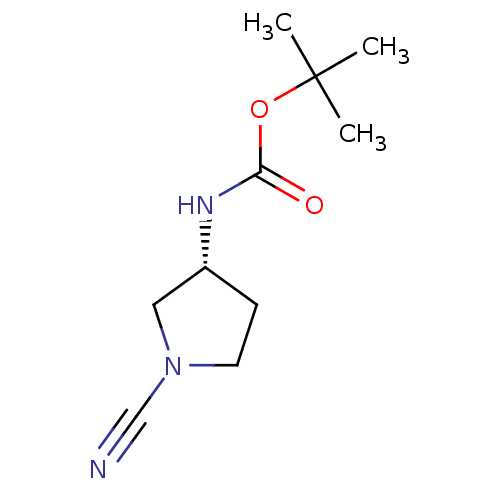
(CHEMBL2096698)Show InChI InChI=1S/C10H17N3O2/c1-10(2,3)15-9(14)12-8-4-5-13(6-8)7-11/h8H,4-6H2,1-3H3,(H,12,14)/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163845

(3-Cyanoamino-pyrrolidine-1-carboxylic acid (S)-1-c...)Show InChI InChI=1S/C16H27N3O2/c1-2-15(10-13-6-4-3-5-7-13)21-16(20)19-9-8-14(11-19)18-12-17/h13-15,18H,2-11H2,1H3/t14?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163839
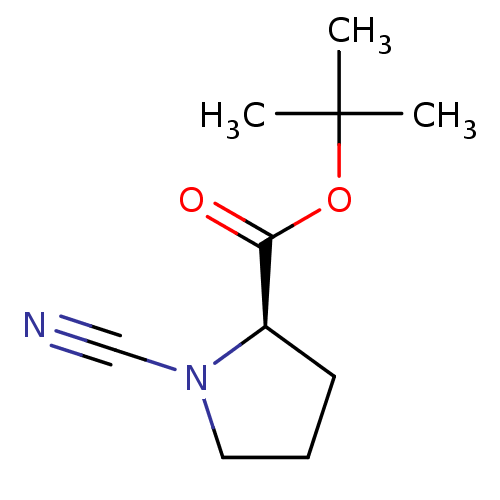
((R)-1-Cyano-pyrrolidine-2-carboxylic acid tert-but...)Show InChI InChI=1S/C10H16N2O2/c1-10(2,3)14-9(13)8-5-4-6-12(8)7-11/h8H,4-6H2,1-3H3/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50163832

((1S)-2-cyclohexyl-1-methylethyl (2S)-1-cyanopyrrol...)Show InChI InChI=1S/C15H24N2O2/c1-12(10-13-6-3-2-4-7-13)19-15(18)14-8-5-9-17(14)11-16/h12-14H,2-10H2,1H3/t12-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin B using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163837

(2-Cyano-pyrazolidine-1-carboxylic acid tert-butyl ...)Show InChI InChI=1S/C9H15N3O2/c1-9(2,3)14-8(13)12-6-4-5-11(12)7-10/h4-6H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163844

((3S,4R)-1-Cyano-4-(3-cyclopentyloxy-4-methoxy-phen...)Show SMILES COC(=O)[C@@H]1CN(C[C@H]1c1ccc(OC)c(OC2CCCC2)c1)C#N Show InChI InChI=1S/C19H24N2O4/c1-23-17-8-7-13(9-18(17)25-14-5-3-4-6-14)15-10-21(12-20)11-16(15)19(22)24-2/h7-9,14-16H,3-6,10-11H2,1-2H3/t15-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Pro-cathepsin H
(Homo sapiens (Human)) | BDBM50163832

((1S)-2-cyclohexyl-1-methylethyl (2S)-1-cyanopyrrol...)Show InChI InChI=1S/C15H24N2O2/c1-12(10-13-6-3-2-4-7-13)19-15(18)14-8-5-9-17(14)11-16/h12-14H,2-10H2,1H3/t12-,14?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin H using 50 uM L-Arg-beta-naphthalamide |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50163838

((1-Cyano-pyrrolidin-3-yl)-carbamic acid (S)-2-cycl...)Show InChI InChI=1S/C15H25N3O2/c1-12(9-13-5-3-2-4-6-13)20-15(19)17-14-7-8-18(10-14)11-16/h12-14H,2-10H2,1H3,(H,17,19)/t12-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin B using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163843
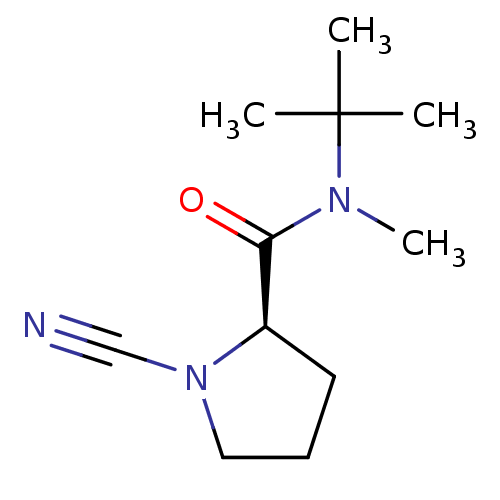
((R)-1-Cyano-pyrrolidine-2-carboxylic acid tert-but...)Show InChI InChI=1S/C11H19N3O/c1-11(2,3)13(4)10(15)9-6-5-7-14(9)8-12/h9H,5-7H2,1-4H3/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163840
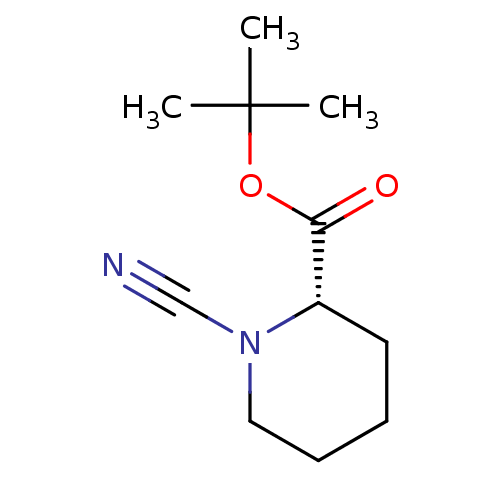
((S)-1-Cyano-piperidine-2-carboxylic acid tert-buty...)Show InChI InChI=1S/C11H18N2O2/c1-11(2,3)15-10(14)9-6-4-5-7-13(9)8-12/h9H,4-7H2,1-3H3/t9-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50095495
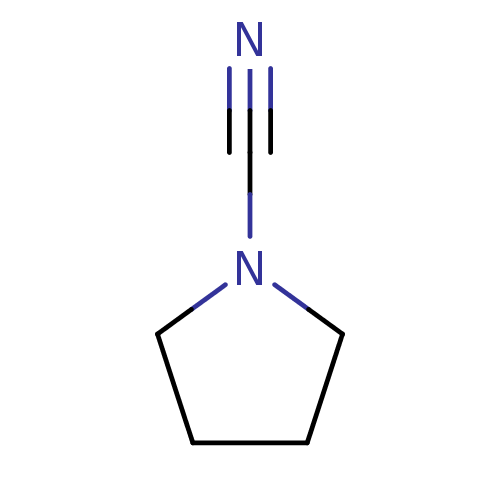
(CHEMBL262697 | Pyrrolidine-1-carbonitrile)Show InChI InChI=1S/C5H8N2/c6-5-7-3-1-2-4-7/h1-4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50163833
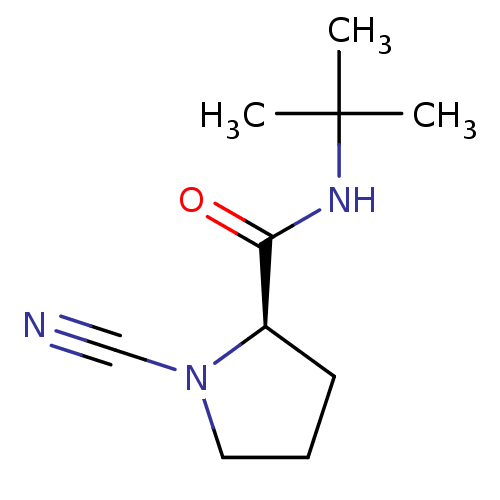
((R)-1-Cyano-pyrrolidine-2-carboxylic acid tert-but...)Show InChI InChI=1S/C10H17N3O/c1-10(2,3)12-9(14)8-5-4-6-13(8)7-11/h8H,4-6H2,1-3H3,(H,12,14)/t8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin K using 10 uM Cbz-Phe-Arg-AMC |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |
Pro-cathepsin H
(Homo sapiens (Human)) | BDBM50163838

((1-Cyano-pyrrolidin-3-yl)-carbamic acid (S)-2-cycl...)Show InChI InChI=1S/C15H25N3O2/c1-12(9-13-5-3-2-4-6-13)20-15(19)17-14-7-8-18(10-14)11-16/h12-14H,2-10H2,1H3,(H,17,19)/t12-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human cathepsin H using 50 uM L-Arg-beta-naphthalamide |
Bioorg Med Chem Lett 15: 1815-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.033
BindingDB Entry DOI: 10.7270/Q2DR2V1G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data