 Found 83 hits of Enzyme Inhibition Constant Data
Found 83 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50006189
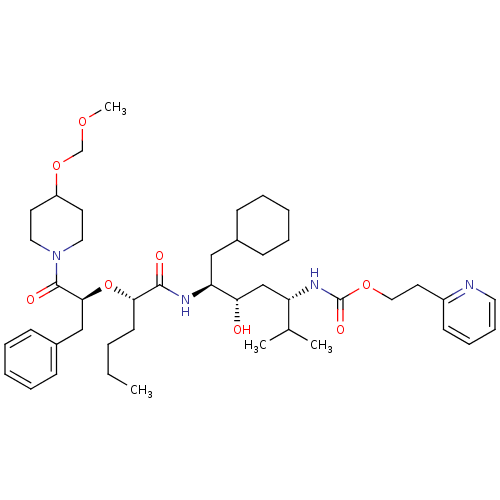
((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCc1ccccn1)C(C)C Show InChI InChI=1S/C44H68N4O8/c1-5-6-20-40(56-41(29-34-17-11-8-12-18-34)43(51)48-25-21-36(22-26-48)55-31-53-4)42(50)46-38(28-33-15-9-7-10-16-33)39(49)30-37(32(2)3)47-44(52)54-27-23-35-19-13-14-24-45-35/h8,11-14,17-19,24,32-33,36-41,49H,5-7,9-10,15-16,20-23,25-31H2,1-4H3,(H,46,50)(H,47,52)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006206
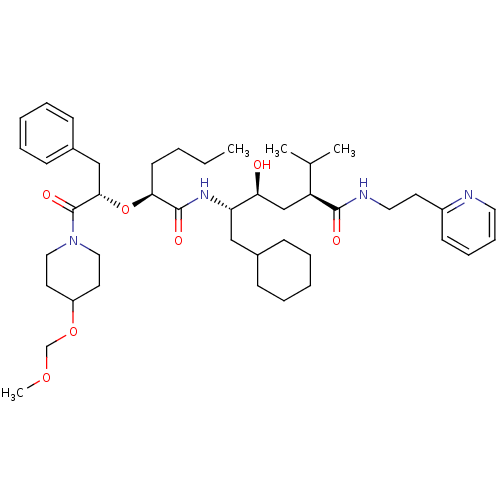
(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCc1ccccn1 Show InChI InChI=1S/C44H68N4O7/c1-5-6-20-40(55-41(29-34-17-11-8-12-18-34)44(52)48-26-22-36(23-27-48)54-31-53-4)43(51)47-38(28-33-15-9-7-10-16-33)39(49)30-37(32(2)3)42(50)46-25-21-35-19-13-14-24-45-35/h8,11-14,17-19,24,32-33,36-41,49H,5-7,9-10,15-16,20-23,25-31H2,1-4H3,(H,46,50)(H,47,51)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006152
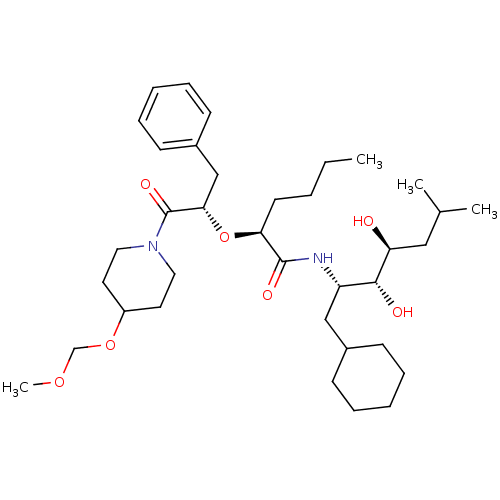
(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H60N2O7/c1-5-6-17-32(35(41)37-30(23-27-13-9-7-10-14-27)34(40)31(39)22-26(2)3)45-33(24-28-15-11-8-12-16-28)36(42)38-20-18-29(19-21-38)44-25-43-4/h8,11-12,15-16,26-27,29-34,39-40H,5-7,9-10,13-14,17-25H2,1-4H3,(H,37,41)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006185
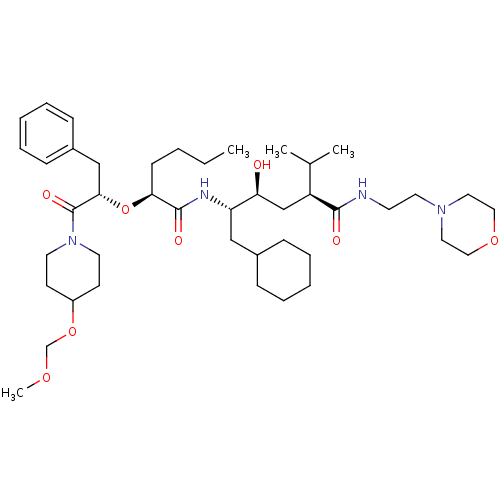
(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C43H72N4O8/c1-5-6-17-39(55-40(29-34-15-11-8-12-16-34)43(51)47-21-18-35(19-22-47)54-31-52-4)42(50)45-37(28-33-13-9-7-10-14-33)38(48)30-36(32(2)3)41(49)44-20-23-46-24-26-53-27-25-46/h8,11-12,15-16,32-33,35-40,48H,5-7,9-10,13-14,17-31H2,1-4H3,(H,44,49)(H,45,50)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006161
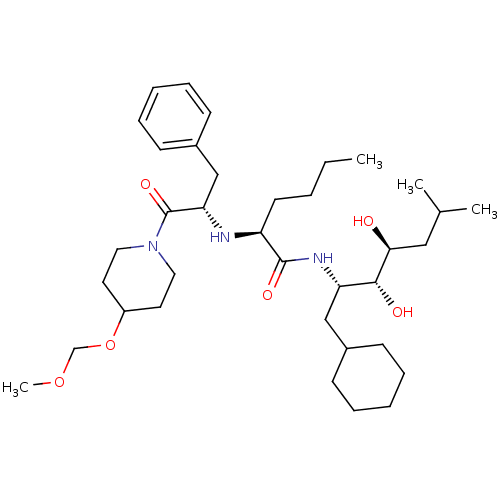
(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H61N3O6/c1-5-6-17-30(35(42)38-31(23-27-13-9-7-10-14-27)34(41)33(40)22-26(2)3)37-32(24-28-15-11-8-12-16-28)36(43)39-20-18-29(19-21-39)45-25-44-4/h8,11-12,15-16,26-27,29-34,37,40-41H,5-7,9-10,13-14,17-25H2,1-4H3,(H,38,42)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006204

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCC[N+]1([O-])CCOCC1 Show InChI InChI=1S/C44H74N4O9/c1-5-6-18-40(57-41(30-35-16-11-8-12-17-35)44(52)47-22-19-36(20-23-47)56-32-54-4)43(51)46-38(29-34-14-9-7-10-15-34)39(49)31-37(33(2)3)42(50)45-21-13-24-48(53)25-27-55-28-26-48/h8,11-12,16-17,33-34,36-41,49H,5-7,9-10,13-15,18-32H2,1-4H3,(H,45,50)(H,46,51)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006211
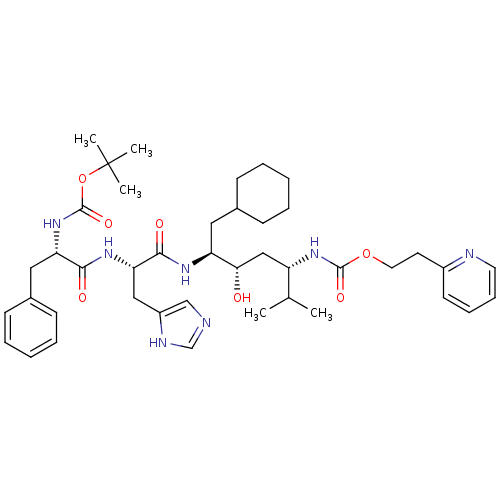
(CHEMBL49218 | {4-[2-(2-tert-Butoxycarbonylamino-3-...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCCc1ccccn1 Show InChI InChI=1S/C42H61N7O7/c1-28(2)33(48-40(53)55-21-19-31-18-12-13-20-44-31)25-37(50)34(22-29-14-8-6-9-15-29)46-39(52)36(24-32-26-43-27-45-32)47-38(51)35(23-30-16-10-7-11-17-30)49-41(54)56-42(3,4)5/h7,10-13,16-18,20,26-29,33-37,50H,6,8-9,14-15,19,21-25H2,1-5H3,(H,43,45)(H,46,52)(H,47,51)(H,48,53)(H,49,54)/t33-,34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006176
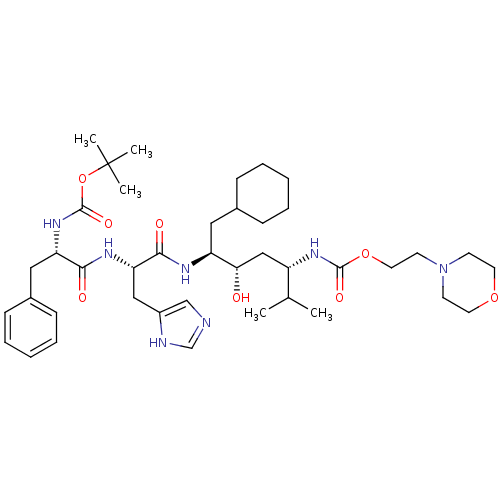
(CHEMBL264194 | {4-[2-(2-tert-Butoxycarbonylamino-3...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCCN1CCOCC1 Show InChI InChI=1S/C41H65N7O8/c1-28(2)32(46-39(52)55-21-18-48-16-19-54-20-17-48)25-36(49)33(22-29-12-8-6-9-13-29)44-38(51)35(24-31-26-42-27-43-31)45-37(50)34(23-30-14-10-7-11-15-30)47-40(53)56-41(3,4)5/h7,10-11,14-15,26-29,32-36,49H,6,8-9,12-13,16-25H2,1-5H3,(H,42,43)(H,44,51)(H,45,50)(H,46,52)(H,47,53)/t32-,33-,34-,35-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006208
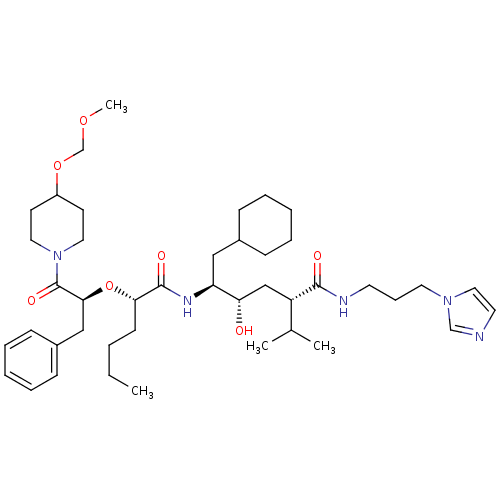
(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCn1ccnc1 Show InChI InChI=1S/C43H69N5O7/c1-5-6-18-39(55-40(28-34-16-11-8-12-17-34)43(52)48-24-19-35(20-25-48)54-31-53-4)42(51)46-37(27-33-14-9-7-10-15-33)38(49)29-36(32(2)3)41(50)45-21-13-23-47-26-22-44-30-47/h8,11-12,16-17,22,26,30,32-33,35-40,49H,5-7,9-10,13-15,18-21,23-25,27-29,31H2,1-4H3,(H,45,50)(H,46,51)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006197

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCC[N+](C)(C)[O-] Show InChI InChI=1S/C42H72N4O8/c1-7-8-20-38(54-39(28-33-18-13-10-14-19-33)42(50)45-24-21-34(22-25-45)53-30-52-6)41(49)44-36(27-32-16-11-9-12-17-32)37(47)29-35(31(2)3)40(48)43-23-15-26-46(4,5)51/h10,13-14,18-19,31-32,34-39,47H,7-9,11-12,15-17,20-30H2,1-6H3,(H,43,48)(H,44,49)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006195
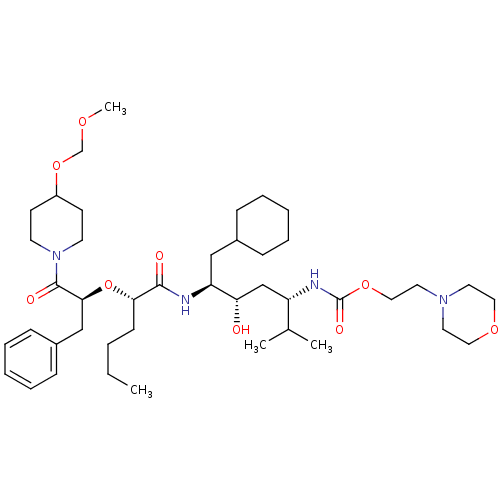
((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCN1CCOCC1)C(C)C Show InChI InChI=1S/C43H72N4O9/c1-5-6-17-39(56-40(29-34-15-11-8-12-16-34)42(50)47-20-18-35(19-21-47)55-31-52-4)41(49)44-37(28-33-13-9-7-10-14-33)38(48)30-36(32(2)3)45-43(51)54-27-24-46-22-25-53-26-23-46/h8,11-12,15-16,32-33,35-40,48H,5-7,9-10,13-14,17-31H2,1-4H3,(H,44,49)(H,45,51)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006148

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCC)O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(C)C Show InChI InChI=1S/C41H69N3O7/c1-6-8-20-37(51-38(27-32-18-14-11-15-19-32)41(48)44-24-21-33(22-25-44)50-29-49-5)40(47)43-35(26-31-16-12-10-13-17-31)36(45)28-34(30(3)4)39(46)42-23-9-7-2/h11,14-15,18-19,30-31,33-38,45H,6-10,12-13,16-17,20-29H2,1-5H3,(H,42,46)(H,43,47)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006184
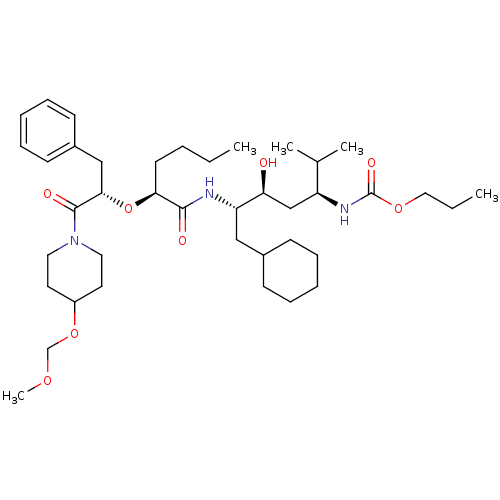
((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCC)C(C)C Show InChI InChI=1S/C40H67N3O8/c1-6-8-19-36(51-37(26-31-17-13-10-14-18-31)39(46)43-22-20-32(21-23-43)50-28-48-5)38(45)41-34(25-30-15-11-9-12-16-30)35(44)27-33(29(3)4)42-40(47)49-24-7-2/h10,13-14,17-18,29-30,32-37,44H,6-9,11-12,15-16,19-28H2,1-5H3,(H,41,45)(H,42,47)/t33-,34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006196
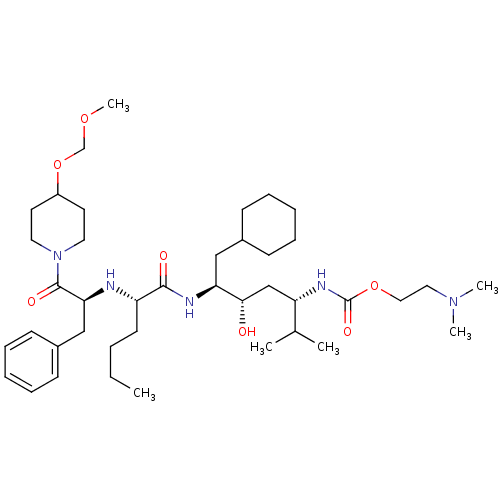
((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCN(C)C)C(C)C Show InChI InChI=1S/C41H71N5O7/c1-7-8-19-34(42-37(27-32-17-13-10-14-18-32)40(49)46-22-20-33(21-23-46)53-29-51-6)39(48)43-36(26-31-15-11-9-12-16-31)38(47)28-35(30(2)3)44-41(50)52-25-24-45(4)5/h10,13-14,17-18,30-31,33-38,42,47H,7-9,11-12,15-16,19-29H2,1-6H3,(H,43,48)(H,44,50)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006207
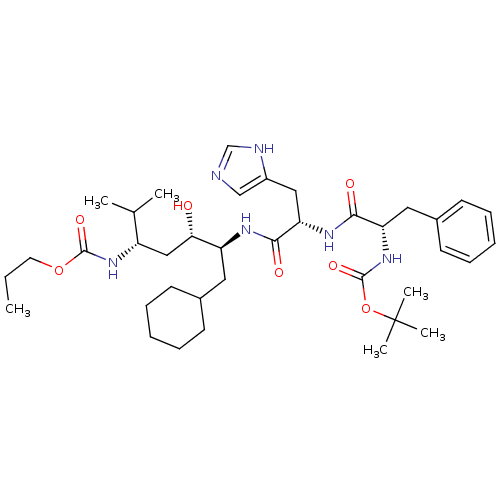
(CHEMBL301395 | {4-[2-(2-tert-Butoxycarbonylamino-3...)Show SMILES CCCOC(=O)N[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C Show InChI InChI=1S/C38H60N6O7/c1-7-18-50-36(48)43-29(25(2)3)22-33(45)30(19-26-14-10-8-11-15-26)41-35(47)32(21-28-23-39-24-40-28)42-34(46)31(20-27-16-12-9-13-17-27)44-37(49)51-38(4,5)6/h9,12-13,16-17,23-26,29-33,45H,7-8,10-11,14-15,18-22H2,1-6H3,(H,39,40)(H,41,47)(H,42,46)(H,43,48)(H,44,49)/t29-,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006157
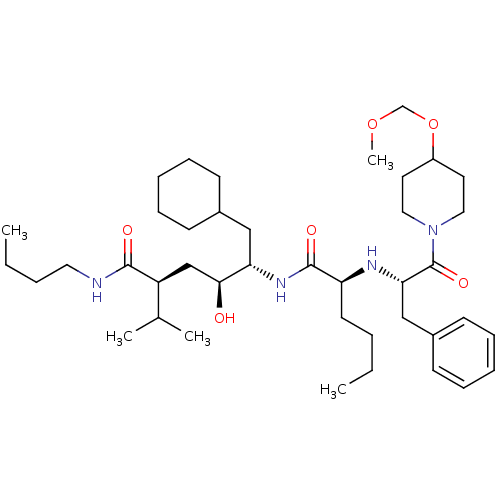
(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCC)N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(C)C Show InChI InChI=1S/C41H70N4O6/c1-6-8-20-35(43-37(27-32-18-14-11-15-19-32)41(49)45-24-21-33(22-25-45)51-29-50-5)40(48)44-36(26-31-16-12-10-13-17-31)38(46)28-34(30(3)4)39(47)42-23-9-7-2/h11,14-15,18-19,30-31,33-38,43,46H,6-10,12-13,16-17,20-29H2,1-5H3,(H,42,47)(H,44,48)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50006200
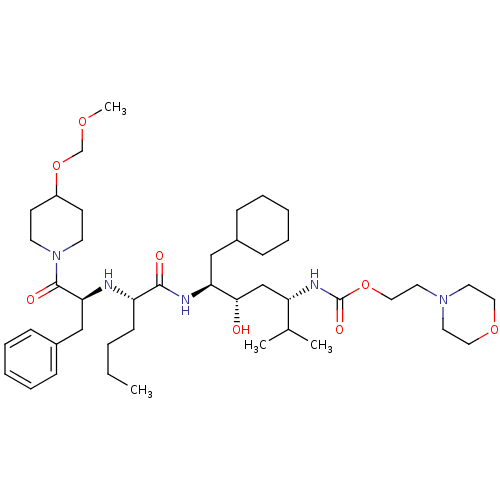
((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCN1CCOCC1)C(C)C Show InChI InChI=1S/C43H73N5O8/c1-5-6-17-36(44-39(29-34-15-11-8-12-16-34)42(51)48-20-18-35(19-21-48)56-31-53-4)41(50)45-38(28-33-13-9-7-10-14-33)40(49)30-37(32(2)3)46-43(52)55-27-24-47-22-25-54-26-23-47/h8,11-12,15-16,32-33,35-40,44,49H,5-7,9-10,13-14,17-31H2,1-4H3,(H,45,50)(H,46,52)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006179

((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCn1ccnc1 Show InChI InChI=1S/C43H70N6O6/c1-5-6-18-37(46-39(28-34-16-11-8-12-17-34)43(53)49-24-19-35(20-25-49)55-31-54-4)42(52)47-38(27-33-14-9-7-10-15-33)40(50)29-36(32(2)3)41(51)45-21-13-23-48-26-22-44-30-48/h8,11-12,16-17,22,26,30,32-33,35-40,46,50H,5-7,9-10,13-15,18-21,23-25,27-29,31H2,1-4H3,(H,45,51)(H,47,52)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006192

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCN(C)C Show InChI InChI=1S/C42H73N5O6/c1-7-8-20-36(44-38(28-33-18-13-10-14-19-33)42(51)47-25-21-34(22-26-47)53-30-52-6)41(50)45-37(27-32-16-11-9-12-17-32)39(48)29-35(31(2)3)40(49)43-23-15-24-46(4)5/h10,13-14,18-19,31-32,34-39,44,48H,7-9,11-12,15-17,20-30H2,1-6H3,(H,43,49)(H,45,50)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50006183
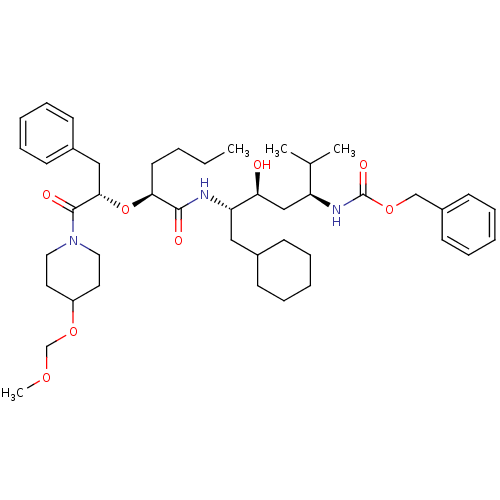
((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C44H67N3O8/c1-5-6-22-40(55-41(28-34-18-12-8-13-19-34)43(50)47-25-23-36(24-26-47)54-31-52-4)42(49)45-38(27-33-16-10-7-11-17-33)39(48)29-37(32(2)3)46-44(51)53-30-35-20-14-9-15-21-35/h8-9,12-15,18-21,32-33,36-41,48H,5-7,10-11,16-17,22-31H2,1-4H3,(H,45,49)(H,46,51)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006202

(3-Amino-N-[1-[1-(1-cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES COc1ccc(C[C@H](NC(=O)CC(C)(C)N)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)[C@@H](O)CC(C)C)cc1 Show InChI InChI=1S/C35H56N6O6/c1-22(2)15-30(42)32(44)27(16-23-9-7-6-8-10-23)40-34(46)29(18-25-20-37-21-38-25)41-33(45)28(39-31(43)19-35(3,4)36)17-24-11-13-26(47-5)14-12-24/h11-14,20-23,27-30,32,42,44H,6-10,15-19,36H2,1-5H3,(H,37,38)(H,39,43)(H,40,46)(H,41,45)/t27-,28-,29-,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.7 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against renin in monkey plasma at pH 7.7 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006213

(CHEMBL48343 | {4-[2-(2-tert-Butoxycarbonylamino-3-...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCCN(C)C Show InChI InChI=1S/C39H63N7O7/c1-26(2)30(44-37(50)52-19-18-46(6)7)23-34(47)31(20-27-14-10-8-11-15-27)42-36(49)33(22-29-24-40-25-41-29)43-35(48)32(21-28-16-12-9-13-17-28)45-38(51)53-39(3,4)5/h9,12-13,16-17,24-27,30-34,47H,8,10-11,14-15,18-23H2,1-7H3,(H,40,41)(H,42,49)(H,43,48)(H,44,50)(H,45,51)/t30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006187
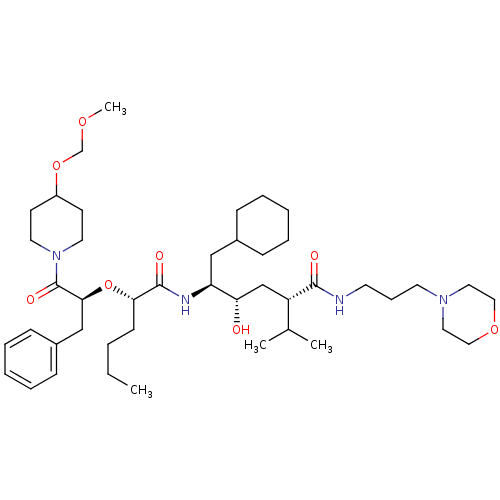
((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCN1CCOCC1 Show InChI InChI=1S/C44H74N4O8/c1-5-6-18-40(56-41(30-35-16-11-8-12-17-35)44(52)48-23-19-36(20-24-48)55-32-53-4)43(51)46-38(29-34-14-9-7-10-15-34)39(49)31-37(33(2)3)42(50)45-21-13-22-47-25-27-54-28-26-47/h8,11-12,16-17,33-34,36-41,49H,5-7,9-10,13-15,18-32H2,1-4H3,(H,45,50)(H,46,51)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006205
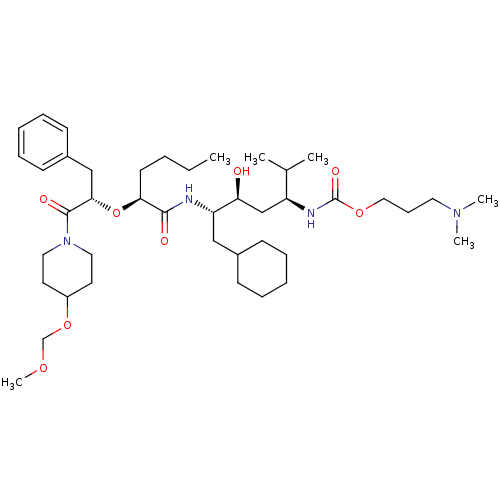
((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCCN(C)C)C(C)C Show InChI InChI=1S/C42H72N4O8/c1-7-8-20-38(54-39(28-33-18-13-10-14-19-33)41(49)46-24-21-34(22-25-46)53-30-51-6)40(48)43-36(27-32-16-11-9-12-17-32)37(47)29-35(31(2)3)44-42(50)52-26-15-23-45(4)5/h10,13-14,18-19,31-32,34-39,47H,7-9,11-12,15-17,20-30H2,1-6H3,(H,43,48)(H,44,50)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006177
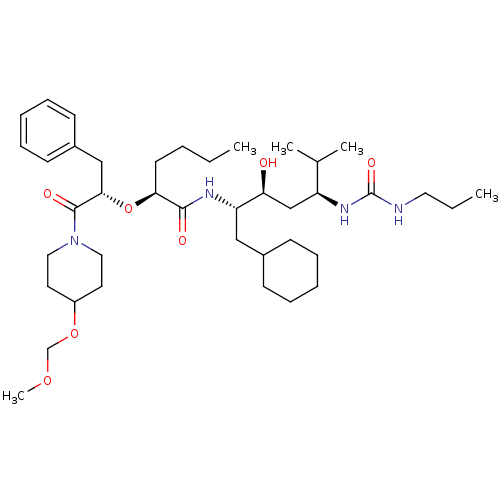
(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)NCCC)C(C)C Show InChI InChI=1S/C40H68N4O7/c1-6-8-19-36(51-37(26-31-17-13-10-14-18-31)39(47)44-23-20-32(21-24-44)50-28-49-5)38(46)42-34(25-30-15-11-9-12-16-30)35(45)27-33(29(3)4)43-40(48)41-22-7-2/h10,13-14,17-18,29-30,32-37,45H,6-9,11-12,15-16,19-28H2,1-5H3,(H,42,46)(H2,41,43,48)/t33-,34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006186
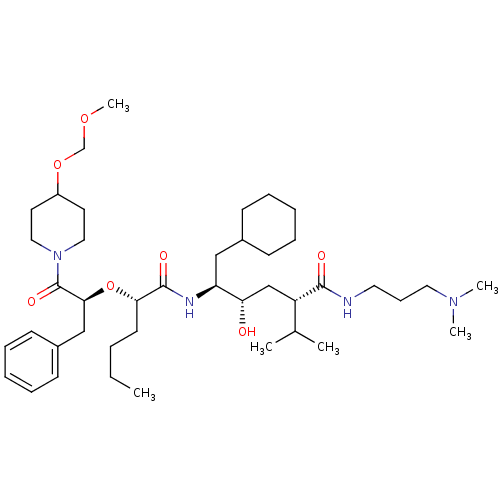
(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCN(C)C Show InChI InChI=1S/C42H72N4O7/c1-7-8-20-38(53-39(28-33-18-13-10-14-19-33)42(50)46-25-21-34(22-26-46)52-30-51-6)41(49)44-36(27-32-16-11-9-12-17-32)37(47)29-35(31(2)3)40(48)43-23-15-24-45(4)5/h10,13-14,18-19,31-32,34-39,47H,7-9,11-12,15-17,20-30H2,1-6H3,(H,43,48)(H,44,49)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006182
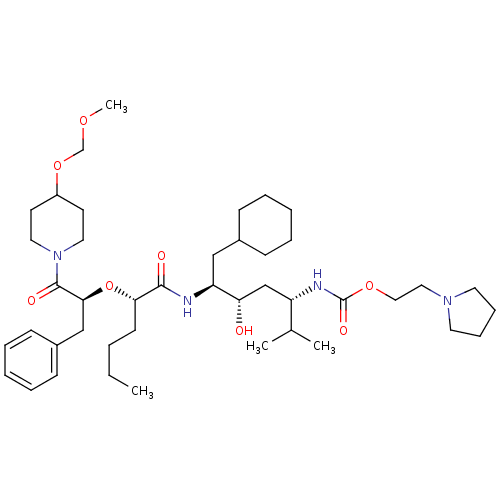
((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCN1CCCC1)C(C)C Show InChI InChI=1S/C43H72N4O8/c1-5-6-19-39(55-40(29-34-17-11-8-12-18-34)42(50)47-24-20-35(21-25-47)54-31-52-4)41(49)44-37(28-33-15-9-7-10-16-33)38(48)30-36(32(2)3)45-43(51)53-27-26-46-22-13-14-23-46/h8,11-12,17-18,32-33,35-40,48H,5-7,9-10,13-16,19-31H2,1-4H3,(H,44,49)(H,45,51)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006214
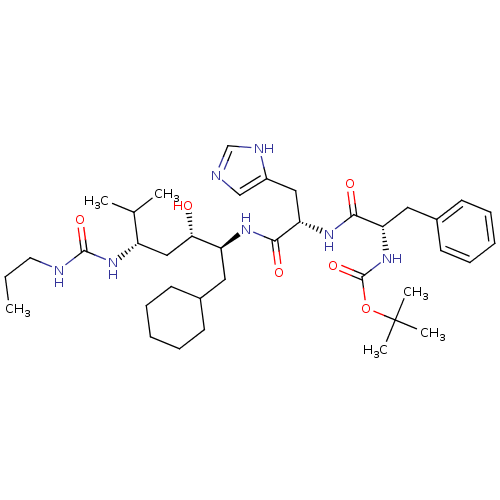
(CHEMBL297701 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...)Show SMILES CCCNC(=O)N[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C Show InChI InChI=1S/C38H61N7O6/c1-7-18-40-36(49)44-29(25(2)3)22-33(46)30(19-26-14-10-8-11-15-26)42-35(48)32(21-28-23-39-24-41-28)43-34(47)31(20-27-16-12-9-13-17-27)45-37(50)51-38(4,5)6/h9,12-13,16-17,23-26,29-33,46H,7-8,10-11,14-15,18-22H2,1-6H3,(H,39,41)(H,42,48)(H,43,47)(H,45,50)(H2,40,44,49)/t29-,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006181

(CHEMBL299605 | {4-[2-(2-tert-Butoxycarbonylamino-3...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCCN1CCCC1 Show InChI InChI=1S/C41H65N7O7/c1-28(2)32(46-39(52)54-21-20-48-18-12-13-19-48)25-36(49)33(22-29-14-8-6-9-15-29)44-38(51)35(24-31-26-42-27-43-31)45-37(50)34(23-30-16-10-7-11-17-30)47-40(53)55-41(3,4)5/h7,10-11,16-17,26-29,32-36,49H,6,8-9,12-15,18-25H2,1-5H3,(H,42,43)(H,44,51)(H,45,50)(H,46,52)(H,47,53)/t32-,33-,34-,35-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006191
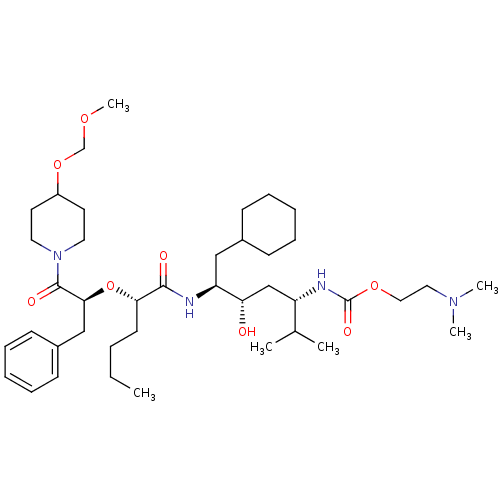
((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCN(C)C)C(C)C Show InChI InChI=1S/C41H70N4O8/c1-7-8-19-37(53-38(27-32-17-13-10-14-18-32)40(48)45-22-20-33(21-23-45)52-29-50-6)39(47)42-35(26-31-15-11-9-12-16-31)36(46)28-34(30(2)3)43-41(49)51-25-24-44(4)5/h10,13-14,17-18,30-31,33-38,46H,7-9,11-12,15-16,19-29H2,1-6H3,(H,42,47)(H,43,49)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006201

(CHEMBL48722 | {4-[2-(2-tert-Butoxycarbonylamino-3-...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCCCN(C)C Show InChI InChI=1S/C40H65N7O7/c1-27(2)31(45-38(51)53-20-14-19-47(6)7)24-35(48)32(21-28-15-10-8-11-16-28)43-37(50)34(23-30-25-41-26-42-30)44-36(49)33(22-29-17-12-9-13-18-29)46-39(52)54-40(3,4)5/h9,12-13,17-18,25-28,31-35,48H,8,10-11,14-16,19-24H2,1-7H3,(H,41,42)(H,43,50)(H,44,49)(H,45,51)(H,46,52)/t31-,32-,33-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006187

((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCN1CCOCC1 Show InChI InChI=1S/C44H74N4O8/c1-5-6-18-40(56-41(30-35-16-11-8-12-17-35)44(52)48-23-19-36(20-24-48)55-32-53-4)43(51)46-38(29-34-14-9-7-10-15-34)39(49)31-37(33(2)3)42(50)45-21-13-22-47-25-27-54-28-26-47/h8,11-12,16-17,33-34,36-41,49H,5-7,9-10,13-15,18-32H2,1-4H3,(H,45,50)(H,46,51)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006180
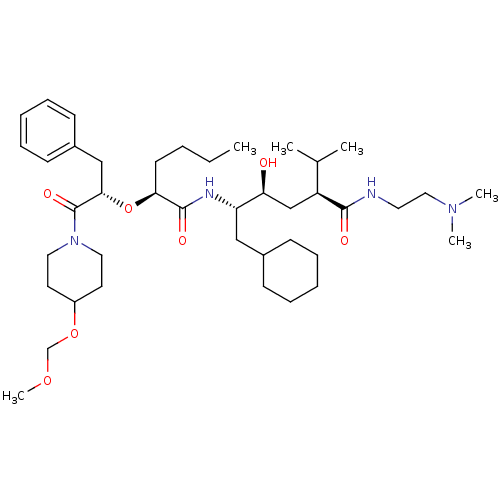
(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCN(C)C Show InChI InChI=1S/C41H70N4O7/c1-7-8-19-37(52-38(27-32-17-13-10-14-18-32)41(49)45-23-20-33(21-24-45)51-29-50-6)40(48)43-35(26-31-15-11-9-12-16-31)36(46)28-34(30(2)3)39(47)42-22-25-44(4)5/h10,13-14,17-18,30-31,33-38,46H,7-9,11-12,15-16,19-29H2,1-6H3,(H,42,47)(H,43,48)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006208

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCn1ccnc1 Show InChI InChI=1S/C43H69N5O7/c1-5-6-18-39(55-40(28-34-16-11-8-12-17-34)43(52)48-24-19-35(20-25-48)54-31-53-4)42(51)46-37(27-33-14-9-7-10-15-33)38(49)29-36(32(2)3)41(50)45-21-13-23-47-26-22-44-30-47/h8,11-12,16-17,22,26,30,32-33,35-40,49H,5-7,9-10,13-15,18-21,23-25,27-29,31H2,1-4H3,(H,45,50)(H,46,51)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006210

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)CCCC)C(C)C Show InChI InChI=1S/C41H69N3O7/c1-6-8-20-37(51-38(27-32-18-14-11-15-19-32)41(48)44-24-22-33(23-25-44)50-29-49-5)40(47)43-35(26-31-16-12-10-13-17-31)36(45)28-34(30(3)4)42-39(46)21-9-7-2/h11,14-15,18-19,30-31,33-38,45H,6-10,12-13,16-17,20-29H2,1-5H3,(H,42,46)(H,43,47)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006198
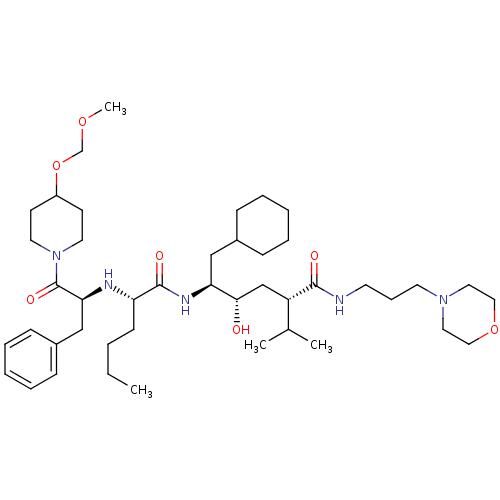
((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCN1CCOCC1 Show InChI InChI=1S/C44H75N5O7/c1-5-6-18-38(46-40(30-35-16-11-8-12-17-35)44(53)49-23-19-36(20-24-49)56-32-54-4)43(52)47-39(29-34-14-9-7-10-15-34)41(50)31-37(33(2)3)42(51)45-21-13-22-48-25-27-55-28-26-48/h8,11-12,16-17,33-34,36-41,46,50H,5-7,9-10,13-15,18-32H2,1-4H3,(H,45,51)(H,47,52)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006204

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCC[N+]1([O-])CCOCC1 Show InChI InChI=1S/C44H74N4O9/c1-5-6-18-40(57-41(30-35-16-11-8-12-17-35)44(52)47-22-19-36(20-23-47)56-32-54-4)43(51)46-38(29-34-14-9-7-10-15-34)39(49)31-37(33(2)3)42(50)45-21-13-24-48(53)25-27-55-28-26-48/h8,11-12,16-17,33-34,36-41,49H,5-7,9-10,13-15,18-32H2,1-4H3,(H,45,50)(H,46,51)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006185

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C43H72N4O8/c1-5-6-17-39(55-40(29-34-15-11-8-12-16-34)43(51)47-21-18-35(19-22-47)54-31-52-4)42(50)45-37(28-33-13-9-7-10-14-33)38(48)30-36(32(2)3)41(49)44-20-23-46-24-26-53-27-25-46/h8,11-12,15-16,32-33,35-40,48H,5-7,9-10,13-14,17-31H2,1-4H3,(H,44,49)(H,45,50)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006178

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NS(C)(=O)=O)C(C)C Show InChI InChI=1S/C37H63N3O8S/c1-6-7-18-34(48-35(24-29-16-12-9-13-17-29)37(43)40-21-19-30(20-22-40)47-26-46-4)36(42)38-32(23-28-14-10-8-11-15-28)33(41)25-31(27(2)3)39-49(5,44)45/h9,12-13,16-17,27-28,30-35,39,41H,6-8,10-11,14-15,18-26H2,1-5H3,(H,38,42)/t31-,32-,33-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006176

(CHEMBL264194 | {4-[2-(2-tert-Butoxycarbonylamino-3...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCCN1CCOCC1 Show InChI InChI=1S/C41H65N7O8/c1-28(2)32(46-39(52)55-21-18-48-16-19-54-20-17-48)25-36(49)33(22-29-12-8-6-9-13-29)44-38(51)35(24-31-26-42-27-43-31)45-37(50)34(23-30-14-10-7-11-15-30)47-40(53)56-41(3,4)5/h7,10-11,14-15,26-29,32-36,49H,6,8-9,12-13,16-25H2,1-5H3,(H,42,43)(H,44,51)(H,45,50)(H,46,52)(H,47,53)/t32-,33-,34-,35-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006197

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCC[N+](C)(C)[O-] Show InChI InChI=1S/C42H72N4O8/c1-7-8-20-38(54-39(28-33-18-13-10-14-19-33)42(50)45-24-21-34(22-25-45)53-30-52-6)41(49)44-36(27-32-16-11-9-12-17-32)37(47)29-35(31(2)3)40(48)43-23-15-26-46(4,5)51/h10,13-14,18-19,31-32,34-39,47H,7-9,11-12,15-17,20-30H2,1-6H3,(H,43,48)(H,44,49)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006212
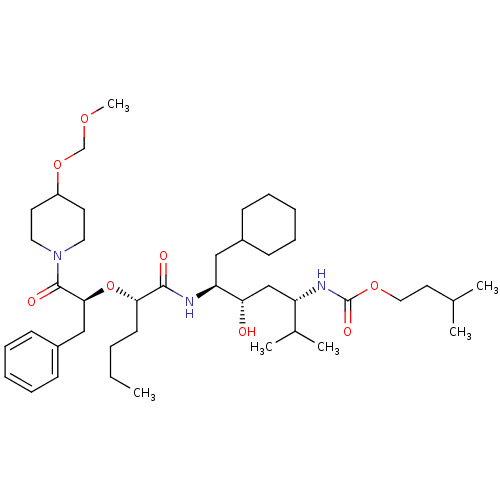
((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCC(C)C)C(C)C Show InChI InChI=1S/C42H71N3O8/c1-7-8-19-38(53-39(27-33-17-13-10-14-18-33)41(48)45-23-20-34(21-24-45)52-29-50-6)40(47)43-36(26-32-15-11-9-12-16-32)37(46)28-35(31(4)5)44-42(49)51-25-22-30(2)3/h10,13-14,17-18,30-32,34-39,46H,7-9,11-12,15-16,19-29H2,1-6H3,(H,43,47)(H,44,49)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006206

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCc1ccccn1 Show InChI InChI=1S/C44H68N4O7/c1-5-6-20-40(55-41(29-34-17-11-8-12-18-34)44(52)48-26-22-36(23-27-48)54-31-53-4)43(51)47-38(28-33-15-9-7-10-16-33)39(49)30-37(32(2)3)42(50)46-25-21-35-19-13-14-24-45-35/h8,11-14,17-19,24,32-33,36-41,49H,5-7,9-10,15-16,20-23,25-31H2,1-4H3,(H,46,50)(H,47,51)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006210

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)CCCC)C(C)C Show InChI InChI=1S/C41H69N3O7/c1-6-8-20-37(51-38(27-32-18-14-11-15-19-32)41(48)44-24-22-33(23-25-44)50-29-49-5)40(47)43-35(26-31-16-12-10-13-17-31)36(45)28-34(30(3)4)42-39(46)21-9-7-2/h11,14-15,18-19,30-31,33-38,45H,6-10,12-13,16-17,20-29H2,1-5H3,(H,42,46)(H,43,47)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006180

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCN(C)C Show InChI InChI=1S/C41H70N4O7/c1-7-8-19-37(52-38(27-32-17-13-10-14-18-32)41(49)45-23-20-33(21-24-45)51-29-50-6)40(48)43-35(26-31-15-11-9-12-16-31)36(46)28-34(30(2)3)39(47)42-22-25-44(4)5/h10,13-14,17-18,30-31,33-38,46H,7-9,11-12,15-16,19-29H2,1-6H3,(H,42,47)(H,43,48)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006186

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCN(C)C Show InChI InChI=1S/C42H72N4O7/c1-7-8-20-38(53-39(28-33-18-13-10-14-19-33)42(50)46-25-21-34(22-26-46)52-30-51-6)41(49)44-36(27-32-16-11-9-12-17-32)37(47)29-35(31(2)3)40(48)43-23-15-24-45(4)5/h10,13-14,18-19,31-32,34-39,47H,7-9,11-12,15-17,20-30H2,1-6H3,(H,43,48)(H,44,49)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006194

(CHEMBL48481 | {1-[1-(1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCCCC(=O)N[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C Show InChI InChI=1S/C39H62N6O6/c1-7-8-19-35(47)42-30(26(2)3)23-34(46)31(20-27-15-11-9-12-16-27)43-37(49)33(22-29-24-40-25-41-29)44-36(48)32(21-28-17-13-10-14-18-28)45-38(50)51-39(4,5)6/h10,13-14,17-18,24-27,30-34,46H,7-9,11-12,15-16,19-23H2,1-6H3,(H,40,41)(H,42,47)(H,43,49)(H,44,48)(H,45,50)/t30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006192

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCN(C)C Show InChI InChI=1S/C42H73N5O6/c1-7-8-20-36(44-38(28-33-18-13-10-14-19-33)42(51)47-25-21-34(22-26-47)53-30-52-6)41(50)45-37(27-32-16-11-9-12-17-32)39(48)29-35(31(2)3)40(49)43-23-15-24-46(4)5/h10,13-14,18-19,31-32,34-39,44,48H,7-9,11-12,15-17,20-30H2,1-6H3,(H,43,49)(H,45,50)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50006198

((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCN1CCOCC1 Show InChI InChI=1S/C44H75N5O7/c1-5-6-18-38(46-40(30-35-16-11-8-12-17-35)44(53)49-23-19-36(20-24-49)56-32-54-4)43(52)47-39(29-34-14-9-7-10-15-34)41(50)31-37(33(2)3)42(51)45-21-13-22-48-25-27-55-28-26-48/h8,11-12,16-17,33-34,36-41,46,50H,5-7,9-10,13-15,18-32H2,1-4H3,(H,45,51)(H,47,52)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006195

((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCN1CCOCC1)C(C)C Show InChI InChI=1S/C43H72N4O9/c1-5-6-17-39(56-40(29-34-15-11-8-12-16-34)42(50)47-20-18-35(19-21-47)55-31-52-4)41(49)44-37(28-33-13-9-7-10-14-33)38(48)30-36(32(2)3)45-43(51)54-27-24-46-22-25-53-26-23-46/h8,11-12,15-16,32-33,35-40,48H,5-7,9-10,13-14,17-31H2,1-4H3,(H,44,49)(H,45,51)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006179

((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)NCCCn1ccnc1 Show InChI InChI=1S/C43H70N6O6/c1-5-6-18-37(46-39(28-34-16-11-8-12-17-34)43(53)49-24-19-35(20-25-49)55-31-54-4)42(52)47-38(27-33-14-9-7-10-15-33)40(50)29-36(32(2)3)41(51)45-21-13-23-48-26-22-44-30-48/h8,11-12,16-17,22,26,30,32-33,35-40,46,50H,5-7,9-10,13-15,18-21,23-25,27-29,31H2,1-4H3,(H,45,51)(H,47,52)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006148

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCC)O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(C)C Show InChI InChI=1S/C41H69N3O7/c1-6-8-20-37(51-38(27-32-18-14-11-15-19-32)41(48)44-24-21-33(22-25-44)50-29-49-5)40(47)43-35(26-31-16-12-10-13-17-31)36(45)28-34(30(3)4)39(46)42-23-9-7-2/h11,14-15,18-19,30-31,33-38,45H,6-10,12-13,16-17,20-29H2,1-5H3,(H,42,46)(H,43,47)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006191

((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCN(C)C)C(C)C Show InChI InChI=1S/C41H70N4O8/c1-7-8-19-37(53-38(27-32-17-13-10-14-18-32)40(48)45-22-20-33(21-23-45)52-29-50-6)39(47)42-35(26-31-15-11-9-12-16-31)36(46)28-34(30(2)3)43-41(49)51-25-24-44(4)5/h10,13-14,17-18,30-31,33-38,46H,7-9,11-12,15-16,19-29H2,1-6H3,(H,42,47)(H,43,49)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006157

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCCC)N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(C)C Show InChI InChI=1S/C41H70N4O6/c1-6-8-20-35(43-37(27-32-18-14-11-15-19-32)41(49)45-24-21-33(22-25-45)51-29-50-5)40(48)44-36(26-31-16-12-10-13-17-31)38(46)28-34(30(3)4)39(47)42-23-9-7-2/h11,14-15,18-19,30-31,33-38,43,46H,6-10,12-13,16-17,20-29H2,1-5H3,(H,42,47)(H,44,48)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50006200

((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCN1CCOCC1)C(C)C Show InChI InChI=1S/C43H73N5O8/c1-5-6-17-36(44-39(29-34-15-11-8-12-16-34)42(51)48-20-18-35(19-21-48)56-31-53-4)41(50)45-38(28-33-13-9-7-10-14-33)40(49)30-37(32(2)3)46-43(52)55-27-24-47-22-25-54-26-23-47/h8,11-12,15-16,32-33,35-40,44,49H,5-7,9-10,13-14,17-31H2,1-4H3,(H,45,50)(H,46,52)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006175

(CHEMBL299612 | {4-[2-(2-tert-Butoxycarbonylamino-3...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C42H60N6O7/c1-28(2)33(47-40(52)54-26-31-19-13-8-14-20-31)24-37(49)34(21-29-15-9-6-10-16-29)45-39(51)36(23-32-25-43-27-44-32)46-38(50)35(22-30-17-11-7-12-18-30)48-41(53)55-42(3,4)5/h7-8,11-14,17-20,25,27-29,33-37,49H,6,9-10,15-16,21-24,26H2,1-5H3,(H,43,44)(H,45,51)(H,46,50)(H,47,52)(H,48,53)/t33-,34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006213

(CHEMBL48343 | {4-[2-(2-tert-Butoxycarbonylamino-3-...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCCN(C)C Show InChI InChI=1S/C39H63N7O7/c1-26(2)30(44-37(50)52-19-18-46(6)7)23-34(47)31(20-27-14-10-8-11-15-27)42-36(49)33(22-29-24-40-25-41-29)43-35(48)32(21-28-16-12-9-13-17-28)45-38(51)53-39(3,4)5/h9,12-13,16-17,24-27,30-34,47H,8,10-11,14-15,18-23H2,1-7H3,(H,40,41)(H,42,49)(H,43,48)(H,44,50)(H,45,51)/t30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006205

((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCCN(C)C)C(C)C Show InChI InChI=1S/C42H72N4O8/c1-7-8-20-38(54-39(28-33-18-13-10-14-19-33)41(49)46-24-21-34(22-25-46)53-30-51-6)40(48)43-36(27-32-16-11-9-12-17-32)37(47)29-35(31(2)3)44-42(50)52-26-15-23-45(4)5/h10,13-14,18-19,31-32,34-39,47H,7-9,11-12,15-17,20-30H2,1-6H3,(H,43,48)(H,44,50)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006196

((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCN(C)C)C(C)C Show InChI InChI=1S/C41H71N5O7/c1-7-8-19-34(42-37(27-32-17-13-10-14-18-32)40(49)46-22-20-33(21-23-46)53-29-51-6)39(48)43-36(26-31-15-11-9-12-16-31)38(47)28-35(30(2)3)44-41(50)52-25-24-45(4)5/h10,13-14,17-18,30-31,33-38,42,47H,7-9,11-12,15-16,19-29H2,1-6H3,(H,43,48)(H,44,50)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006182

((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCN1CCCC1)C(C)C Show InChI InChI=1S/C43H72N4O8/c1-5-6-19-39(55-40(29-34-17-11-8-12-18-34)42(50)47-24-20-35(21-25-47)54-31-52-4)41(49)44-37(28-33-15-9-7-10-16-33)38(48)30-36(32(2)3)45-43(51)53-27-26-46-22-13-14-23-46/h8,11-12,17-18,32-33,35-40,48H,5-7,9-10,13-16,19-31H2,1-4H3,(H,44,49)(H,45,51)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006152

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H60N2O7/c1-5-6-17-32(35(41)37-30(23-27-13-9-7-10-14-27)34(40)31(39)22-26(2)3)45-33(24-28-15-11-8-12-16-28)36(42)38-20-18-29(19-21-38)44-25-43-4/h8,11-12,15-16,26-27,29-34,39-40H,5-7,9-10,13-14,17-25H2,1-4H3,(H,37,41)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006184

((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCC)C(C)C Show InChI InChI=1S/C40H67N3O8/c1-6-8-19-36(51-37(26-31-17-13-10-14-18-31)39(46)43-22-20-32(21-23-43)50-28-48-5)38(45)41-34(25-30-15-11-9-12-16-30)35(44)27-33(29(3)4)42-40(47)49-24-7-2/h10,13-14,17-18,29-30,32-37,44H,6-9,11-12,15-16,19-28H2,1-5H3,(H,41,45)(H,42,47)/t33-,34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006207

(CHEMBL301395 | {4-[2-(2-tert-Butoxycarbonylamino-3...)Show SMILES CCCOC(=O)N[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C Show InChI InChI=1S/C38H60N6O7/c1-7-18-50-36(48)43-29(25(2)3)22-33(45)30(19-26-14-10-8-11-15-26)41-35(47)32(21-28-23-39-24-40-28)42-34(46)31(20-27-16-12-9-13-17-27)44-37(49)51-38(4,5)6/h9,12-13,16-17,23-26,29-33,45H,7-8,10-11,14-15,18-22H2,1-6H3,(H,39,40)(H,41,47)(H,42,46)(H,43,48)(H,44,49)/t29-,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006177

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)NCCC)C(C)C Show InChI InChI=1S/C40H68N4O7/c1-6-8-19-36(51-37(26-31-17-13-10-14-18-31)39(47)44-23-20-32(21-24-44)50-28-49-5)38(46)42-34(25-30-15-11-9-12-16-30)35(45)27-33(29(3)4)43-40(48)41-22-7-2/h10,13-14,17-18,29-30,32-37,45H,6-9,11-12,15-16,19-28H2,1-5H3,(H,42,46)(H2,41,43,48)/t33-,34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006201

(CHEMBL48722 | {4-[2-(2-tert-Butoxycarbonylamino-3-...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCCCN(C)C Show InChI InChI=1S/C40H65N7O7/c1-27(2)31(45-38(51)53-20-14-19-47(6)7)24-35(48)32(21-28-15-10-8-11-16-28)43-37(50)34(23-30-25-41-26-42-30)44-36(49)33(22-29-17-12-9-13-18-29)46-39(52)54-40(3,4)5/h9,12-13,17-18,25-28,31-35,48H,8,10-11,14-16,19-24H2,1-7H3,(H,41,42)(H,43,50)(H,44,49)(H,45,51)(H,46,52)/t31-,32-,33-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006161

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](N[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C36H61N3O6/c1-5-6-17-30(35(42)38-31(23-27-13-9-7-10-14-27)34(41)33(40)22-26(2)3)37-32(24-28-15-11-8-12-16-28)36(43)39-20-18-29(19-21-39)45-25-44-4/h8,11-12,15-16,26-27,29-34,37,40-41H,5-7,9-10,13-14,17-25H2,1-4H3,(H,38,42)/t30-,31-,32-,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006190
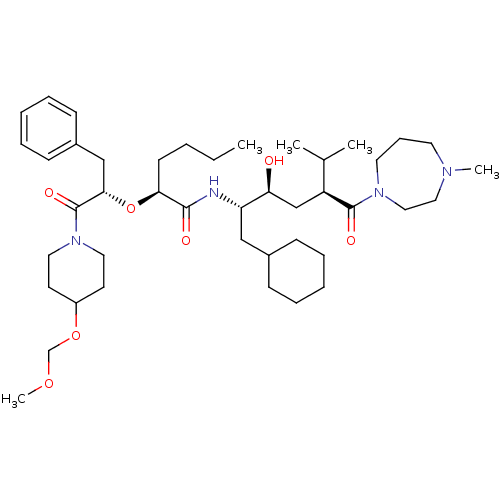
(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)N1CCCN(C)CC1 Show InChI InChI=1S/C43H72N4O7/c1-6-7-19-39(54-40(29-34-17-12-9-13-18-34)43(51)47-24-20-35(21-25-47)53-31-52-5)41(49)44-37(28-33-15-10-8-11-16-33)38(48)30-36(32(2)3)42(50)46-23-14-22-45(4)26-27-46/h9,12-13,17-18,32-33,35-40,48H,6-8,10-11,14-16,19-31H2,1-5H3,(H,44,49)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006211

(CHEMBL49218 | {4-[2-(2-tert-Butoxycarbonylamino-3-...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCCc1ccccn1 Show InChI InChI=1S/C42H61N7O7/c1-28(2)33(48-40(53)55-21-19-31-18-12-13-20-44-31)25-37(50)34(22-29-14-8-6-9-15-29)46-39(52)36(24-32-26-43-27-45-32)47-38(51)35(23-30-16-10-7-11-17-30)49-41(54)56-42(3,4)5/h7,10-13,16-18,20,26-29,33-37,50H,6,8-9,14-15,19,21-25H2,1-5H3,(H,43,45)(H,46,52)(H,47,51)(H,48,53)(H,49,54)/t33-,34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006203
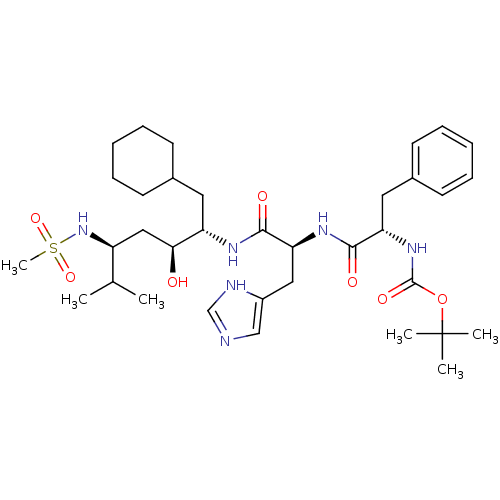
(CHEMBL49882 | {1-[1-(1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NS(C)(=O)=O Show InChI InChI=1S/C35H56N6O7S/c1-23(2)27(41-49(6,46)47)20-31(42)28(17-24-13-9-7-10-14-24)38-33(44)30(19-26-21-36-22-37-26)39-32(43)29(18-25-15-11-8-12-16-25)40-34(45)48-35(3,4)5/h8,11-12,15-16,21-24,27-31,41-42H,7,9-10,13-14,17-20H2,1-6H3,(H,36,37)(H,38,44)(H,39,43)(H,40,45)/t27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006181

(CHEMBL299605 | {4-[2-(2-tert-Butoxycarbonylamino-3...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NC(=O)OCCN1CCCC1 Show InChI InChI=1S/C41H65N7O7/c1-28(2)32(46-39(52)54-21-20-48-18-12-13-19-48)25-36(49)33(22-29-14-8-6-9-15-29)44-38(51)35(24-31-26-42-27-43-31)45-37(50)34(23-30-16-10-7-11-17-30)47-40(53)55-41(3,4)5/h7,10-11,16-17,26-29,32-36,49H,6,8-9,12-15,18-25H2,1-5H3,(H,42,43)(H,44,51)(H,45,50)(H,46,52)(H,47,53)/t32-,33-,34-,35-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006199
(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)N(C)CCN(C)C Show InChI InChI=1S/C42H72N4O7/c1-8-9-20-38(53-39(28-33-18-14-11-15-19-33)42(50)46-23-21-34(22-24-46)52-30-51-7)40(48)43-36(27-32-16-12-10-13-17-32)37(47)29-35(31(2)3)41(49)45(6)26-25-44(4)5/h11,14-15,18-19,31-32,34-39,47H,8-10,12-13,16-17,20-30H2,1-7H3,(H,43,48)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006189

((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCc1ccccn1)C(C)C Show InChI InChI=1S/C44H68N4O8/c1-5-6-20-40(56-41(29-34-17-11-8-12-18-34)43(51)48-25-21-36(22-26-48)55-31-53-4)42(50)46-38(28-33-15-9-7-10-16-33)39(49)30-37(32(2)3)47-44(52)54-27-23-35-19-13-14-24-45-35/h8,11-14,17-19,24,32-33,36-41,49H,5-7,9-10,15-16,20-23,25-31H2,1-4H3,(H,46,50)(H,47,52)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006212

((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCCC(C)C)C(C)C Show InChI InChI=1S/C42H71N3O8/c1-7-8-19-38(53-39(27-33-17-13-10-14-18-33)41(48)45-23-20-34(21-24-45)52-29-50-6)40(47)43-36(26-32-15-11-9-12-16-32)37(46)28-35(31(4)5)44-42(49)51-25-22-30(2)3/h10,13-14,17-18,30-32,34-39,46H,7-9,11-12,15-16,19-29H2,1-6H3,(H,43,47)(H,44,49)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006193
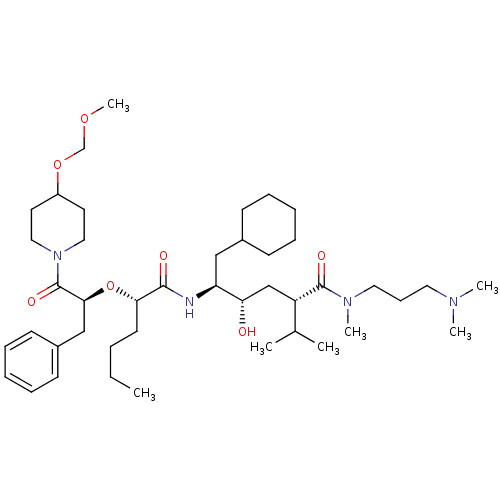
(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)N(C)CCCN(C)C Show InChI InChI=1S/C43H74N4O7/c1-8-9-21-39(54-40(29-34-19-14-11-15-20-34)43(51)47-26-22-35(23-27-47)53-31-52-7)41(49)44-37(28-33-17-12-10-13-18-33)38(48)30-36(32(2)3)42(50)46(6)25-16-24-45(4)5/h11,14-15,19-20,32-33,35-40,48H,8-10,12-13,16-18,21-31H2,1-7H3,(H,44,49)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006178

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NS(C)(=O)=O)C(C)C Show InChI InChI=1S/C37H63N3O8S/c1-6-7-18-34(48-35(24-29-16-12-9-13-17-29)37(43)40-21-19-30(20-22-40)47-26-46-4)36(42)38-32(23-28-14-10-8-11-15-28)33(41)25-31(27(2)3)39-49(5,44)45/h9,12-13,16-17,27-28,30-35,39,41H,6-8,10-11,14-15,18-26H2,1-5H3,(H,38,42)/t31-,32-,33-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006188

(CHEMBL300504 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...)Show SMILES CC(C)[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)NS(=O)(=O)C(C)C Show InChI InChI=1S/C37H60N6O7S/c1-24(2)29(43-51(48,49)25(3)4)21-33(44)30(18-26-14-10-8-11-15-26)40-35(46)32(20-28-22-38-23-39-28)41-34(45)31(19-27-16-12-9-13-17-27)42-36(47)50-37(5,6)7/h9,12-13,16-17,22-26,29-33,43-44H,8,10-11,14-15,18-21H2,1-7H3,(H,38,39)(H,40,46)(H,41,45)(H,42,47)/t29-,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006190

(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)N1CCCN(C)CC1 Show InChI InChI=1S/C43H72N4O7/c1-6-7-19-39(54-40(29-34-17-12-9-13-18-34)43(51)47-24-20-35(21-25-47)53-31-52-5)41(49)44-37(28-33-15-10-8-11-16-33)38(48)30-36(32(2)3)42(50)46-23-14-22-45(4)26-27-46/h9,12-13,17-18,32-33,35-40,48H,6-8,10-11,14-16,19-31H2,1-5H3,(H,44,49)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006183

((4-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C44H67N3O8/c1-5-6-22-40(55-41(28-34-18-12-8-13-19-34)43(50)47-25-23-36(24-26-47)54-31-52-4)42(49)45-38(27-33-16-10-7-11-17-33)39(48)29-37(32(2)3)46-44(51)53-30-35-20-14-9-15-21-35/h8-9,12-15,18-21,32-33,36-41,48H,5-7,10-11,16-17,22-31H2,1-4H3,(H,45,49)(H,46,51)/t37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006214

(CHEMBL297701 | {1-[1-[1-Cyclohexylmethyl-2-hydroxy...)Show SMILES CCCNC(=O)N[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C Show InChI InChI=1S/C38H61N7O6/c1-7-18-40-36(49)44-29(25(2)3)22-33(46)30(19-26-14-10-8-11-15-26)42-35(48)32(21-28-23-39-24-41-28)43-34(47)31(20-27-16-12-9-13-17-27)45-37(50)51-38(4,5)6/h9,12-13,16-17,23-26,29-33,46H,7-8,10-11,14-15,18-22H2,1-6H3,(H,39,41)(H,42,48)(H,43,47)(H,45,50)(H2,40,44,49)/t29-,30-,31-,32-,33-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006209
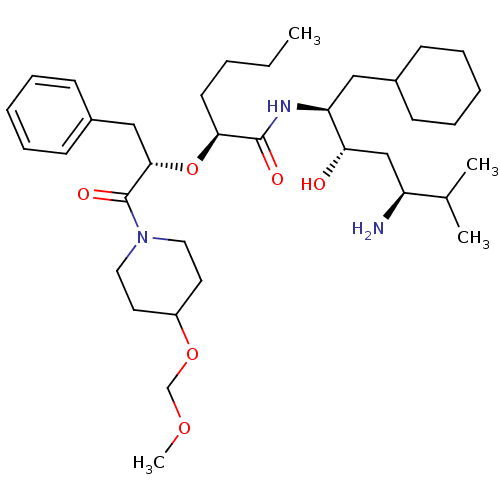
(2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)-2-...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@H](N)C(C)C Show InChI InChI=1S/C36H61N3O6/c1-5-6-17-33(35(41)38-31(22-27-13-9-7-10-14-27)32(40)24-30(37)26(2)3)45-34(23-28-15-11-8-12-16-28)36(42)39-20-18-29(19-21-39)44-25-43-4/h8,11-12,15-16,26-27,29-34,40H,5-7,9-10,13-14,17-25,37H2,1-4H3,(H,38,41)/t30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro activity against human renin (pH 6.0) |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006194

(CHEMBL48481 | {1-[1-(1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCCCC(=O)N[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(C)C Show InChI InChI=1S/C39H62N6O6/c1-7-8-19-35(47)42-30(26(2)3)23-34(46)31(20-27-15-11-9-12-16-27)43-37(49)33(22-29-24-40-25-41-29)44-36(48)32(21-28-17-13-10-14-18-28)45-38(50)51-39(4,5)6/h10,13-14,17-18,24-27,30-34,46H,7-9,11-12,15-16,19-23H2,1-6H3,(H,40,41)(H,42,47)(H,43,49)(H,44,48)(H,45,50)/t30-,31-,32-,33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006193

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)N(C)CCCN(C)C Show InChI InChI=1S/C43H74N4O7/c1-8-9-21-39(54-40(29-34-19-14-11-15-20-34)43(51)47-26-22-35(23-27-47)53-31-52-7)41(49)44-37(28-33-17-12-10-13-18-33)38(48)30-36(32(2)3)42(50)46(6)25-16-24-45(4)5/h11,14-15,19-20,32-33,35-40,48H,8-10,12-13,16-18,21-31H2,1-7H3,(H,44,49)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50006199

(5-{2-[1-Benzyl-2-(4-methoxymethoxy-piperidin-1-yl)...)Show SMILES CCCC[C@H](O[C@@H](Cc1ccccc1)C(=O)N1CCC(CC1)OCOC)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)C[C@@H](C(C)C)C(=O)N(C)CCN(C)C Show InChI InChI=1S/C42H72N4O7/c1-8-9-20-38(53-39(28-33-18-14-11-15-19-33)42(50)46-23-21-34(22-24-46)52-30-51-7)40(48)43-36(27-32-16-12-10-13-17-32)37(47)29-35(31(2)3)41(49)45(6)26-25-44(4)5/h11,14-15,18-19,31-32,34-39,47H,8-10,12-13,16-17,20-30H2,1-7H3,(H,43,48)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro potency against human plasma renin at a pH of 7.4 |
J Med Chem 35: 1735-46 (1992)
BindingDB Entry DOI: 10.7270/Q27943MQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data