

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10692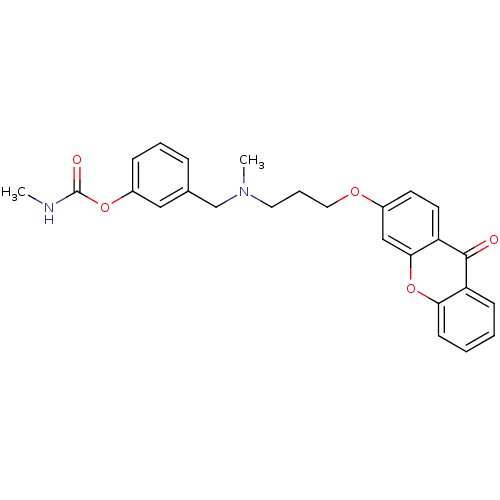 (3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10693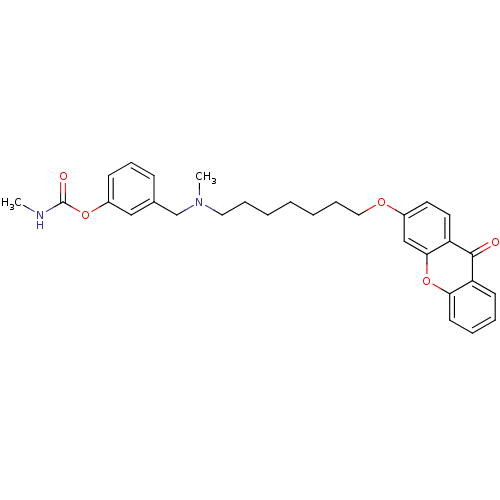 (3-{[methyl({7-[(9-oxo-9H-xanthen-3-yl)oxy]heptyl})...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10697 (3-{[methyl(7-{[(2Z)-3-oxo-2-(3,4,5-trimethoxybenzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10704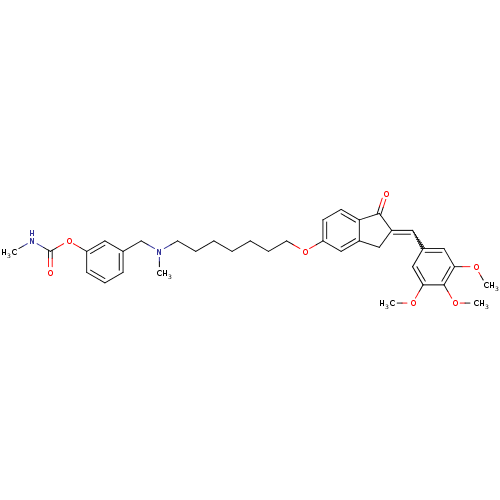 (3-{[methyl(7-{[(2E)-1-oxo-2-(3,4,5-trimethoxybenzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10701 (3-{[(7-{[(2Z)-2-benzylidene-3-oxo-2,3-dihydro-1-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10699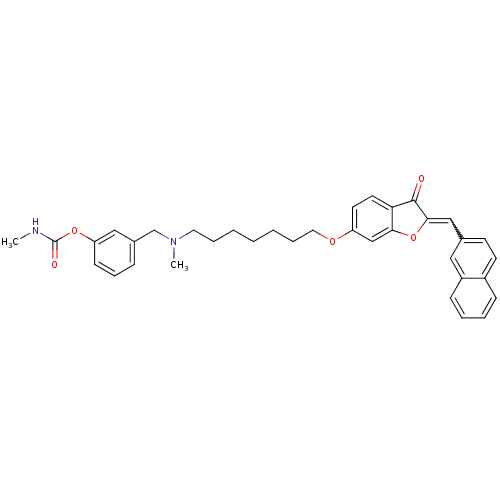 (3-{[methyl(7-{[(2Z)-2-(2-naphthylmethylene)-3-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.09 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10698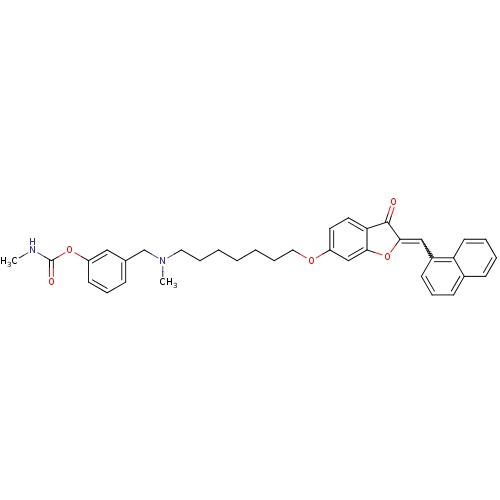 (3-{[methyl(7-{[(2Z)-2-(1-naphthylmethylene)-3-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10694 (3-{[methyl(3-{[(2Z)-3-oxo-2-(3,4,5-trimethoxybenzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.99 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10696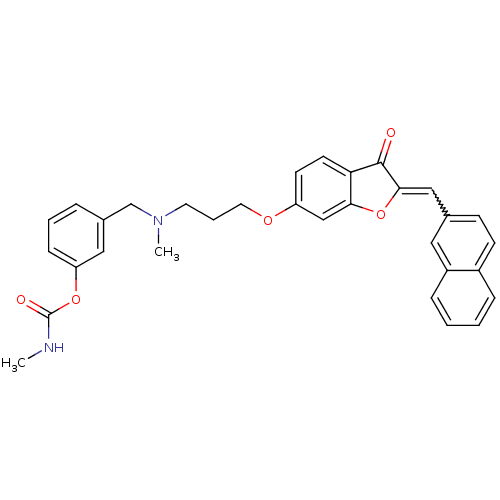 (3-{[methyl(3-{[(2Z)-2-(2-naphthylmethylene)-3-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10706 (3-{[methyl(7-{[(6E)-5-oxo-6-(3,4,5-trimethoxybenzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.94 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10695 (3-{[methyl(3-{[(2Z)-2-(1-naphthylmethylene)-3-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.86 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10702 (3-{[(7-{[(2Z)-2-[(3,4-dichlorophenyl)methylidene]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10705 (3-{[methyl(7-{[(3E)-4-oxo-3-(3,4,5-trimethoxybenzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.81 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10709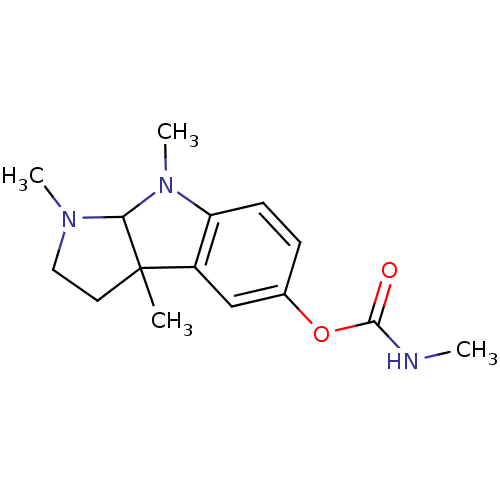 (1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14.1 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10693 (3-{[methyl({7-[(9-oxo-9H-xanthen-3-yl)oxy]heptyl})...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10709 (1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10698 (3-{[methyl(7-{[(2Z)-2-(1-naphthylmethylene)-3-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10695 (3-{[methyl(3-{[(2Z)-2-(1-naphthylmethylene)-3-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10703 (3-{[(7-{[(2Z)-2-(3,5-dichlorobenzylidene)-3-oxo-2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44.6 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10692 (3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10701 (3-{[(7-{[(2Z)-2-benzylidene-3-oxo-2,3-dihydro-1-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10696 (3-{[methyl(3-{[(2Z)-2-(2-naphthylmethylene)-3-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10699 (3-{[methyl(7-{[(2Z)-2-(2-naphthylmethylene)-3-oxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74.8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10700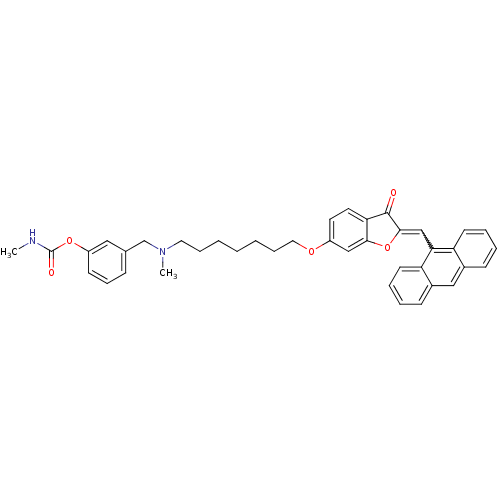 (3-{[(7-{[(2Z)-2-(9-anthrylmethylene)-3-oxo-2,3-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79.1 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10704 (3-{[methyl(7-{[(2E)-1-oxo-2-(3,4,5-trimethoxybenzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10706 (3-{[methyl(7-{[(6E)-5-oxo-6-(3,4,5-trimethoxybenzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84.4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10702 (3-{[(7-{[(2Z)-2-[(3,4-dichlorophenyl)methylidene]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94.6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10703 (3-{[(7-{[(2Z)-2-(3,5-dichlorobenzylidene)-3-oxo-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10697 (3-{[methyl(7-{[(2Z)-3-oxo-2-(3,4,5-trimethoxybenzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10705 (3-{[methyl(7-{[(3E)-4-oxo-3-(3,4,5-trimethoxybenzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10694 (3-{[methyl(3-{[(2Z)-3-oxo-2-(3,4,5-trimethoxybenzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10700 (3-{[(7-{[(2Z)-2-(9-anthrylmethylene)-3-oxo-2,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 761 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10708 (3-[(methyl{7-[(3-oxo-2,3-dihydro-1-benzofuran-6-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10707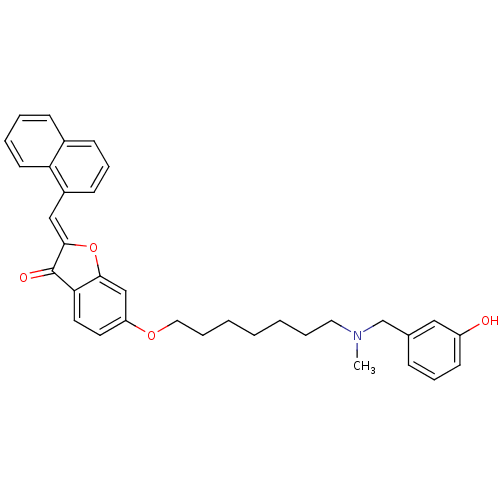 ((2Z)-6-({7-[(3-hydroxybenzyl)(methyl)amino]heptyl}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10708 (3-[(methyl{7-[(3-oxo-2,3-dihydro-1-benzofuran-6-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10707 ((2Z)-6-({7-[(3-hydroxybenzyl)(methyl)amino]heptyl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||