 Found 51 hits of Enzyme Inhibition Constant Data
Found 51 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170763
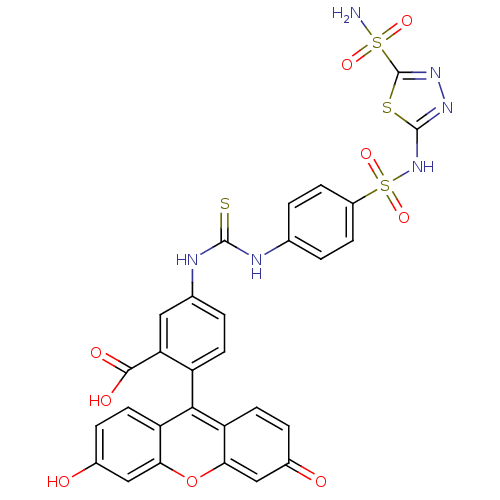
(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-{3-[4-(5-sul...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)Nc3ccc(c(c3)C(O)=O)-c3c4ccc(O)cc4oc4cc(=O)ccc34)cc2)s1 |(32.81,3.4,;31.27,3.4,;31.25,4.94,;31.25,1.86,;29.73,3.24,;28.96,1.89,;27.46,2.21,;27.3,3.73,;25.97,4.5,;24.62,3.71,;25.41,2.37,;23.83,5.06,;23.27,2.92,;21.94,3.69,;20.61,2.92,;20.61,1.38,;19.27,.61,;17.94,1.39,;17.95,2.93,;16.6,.62,;16.59,-.92,;15.24,-1.69,;15.24,-3.23,;16.59,-4,;17.92,-3.23,;17.92,-1.69,;19.26,-4.02,;20.59,-4.79,;20.59,-3.25,;16.59,-5.55,;15.26,-6.32,;13.93,-5.55,;12.58,-6.32,;12.58,-7.86,;11.25,-8.64,;13.93,-8.63,;15.26,-7.87,;16.59,-8.63,;17.93,-7.86,;19.26,-8.63,;20.59,-7.86,;21.94,-8.64,;20.59,-6.32,;19.26,-5.55,;17.93,-6.32,;21.94,.6,;23.27,1.37,;28.7,4.38,)| Show InChI InChI=1S/C29H20N6O9S4/c30-47(40,41)29-34-33-28(46-29)35-48(42,43)18-6-1-14(2-7-18)31-27(45)32-15-3-8-19(22(11-15)26(38)39)25-20-9-4-16(36)12-23(20)44-24-13-17(37)5-10-21(24)25/h1-13,36H,(H,33,35)(H,38,39)(H2,30,40,41)(H2,31,32,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170758

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-{3-[4-(4-sul...)Show SMILES NS(=O)(=O)c1ccc(CNS(=O)(=O)c2ccc(NC(=S)Nc3ccc(c(c3)C(O)=O)-c3c4ccc(O)cc4oc4cc(=O)ccc34)cc2)cc1 |(12.46,-6.66,;12.47,-5.12,;10.93,-5.11,;14.01,-5.12,;12.48,-3.58,;11.14,-2.81,;11.14,-1.27,;12.48,-.5,;12.47,1.04,;11.14,1.81,;9.8,1.02,;9.01,2.37,;10.58,-.33,;8.45,.23,;7.12,1,;5.79,.23,;5.79,-1.31,;4.44,-2.08,;3.11,-1.31,;3.13,.23,;1.78,-2.06,;1.76,-3.6,;.43,-4.37,;.43,-5.93,;1.76,-6.7,;3.09,-5.93,;3.09,-4.39,;4.43,-6.7,;5.76,-7.47,;5.76,-5.93,;1.76,-8.24,;.43,-9.01,;-.9,-8.24,;-2.24,-9.01,;-2.24,-10.55,;-3.59,-11.34,;-.9,-11.32,;.43,-10.55,;1.76,-11.32,;3.1,-10.55,;4.43,-11.32,;5.77,-10.55,;7.12,-11.34,;5.77,-9.01,;4.43,-8.24,;3.1,-9.01,;7.12,-2.08,;8.45,-1.31,;13.81,-1.26,;13.81,-2.8,)| Show InChI InChI=1S/C34H26N4O9S3/c35-49(43,44)24-8-1-19(2-9-24)18-36-50(45,46)25-10-3-20(4-11-25)37-34(48)38-21-5-12-26(29(15-21)33(41)42)32-27-13-6-22(39)16-30(27)47-31-17-23(40)7-14-28(31)32/h1-17,36,39H,18H2,(H,41,42)(H2,35,43,44)(H2,37,38,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170754
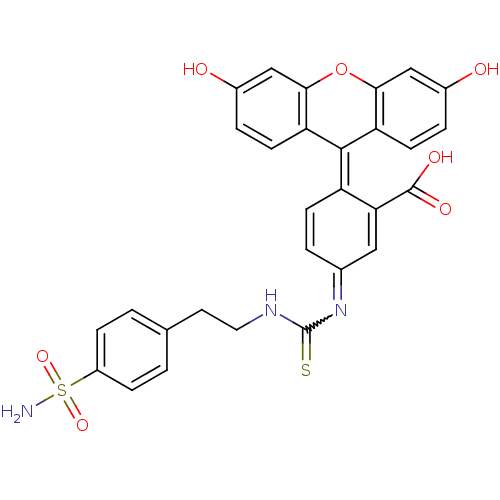
(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-{3-[2-(4-sul...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#6]-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)cc1 |w:13.12,c:15,18| Show InChI InChI=1S/C29H23N3O7S2/c30-41(37,38)20-6-1-16(2-7-20)11-12-31-29(40)32-17-3-8-21(24(13-17)28(35)36)27-22-9-4-18(33)14-25(22)39-26-15-19(34)5-10-23(26)27/h1-10,13-15,33-34H,11-12H2,(H,31,40)(H,35,36)(H2,30,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170756

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(4-sulfam...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)cc1 |w:12.11,c:14,17| Show InChI InChI=1S/C28H21N3O7S2/c29-40(36,37)19-6-1-15(2-7-19)14-30-28(39)31-16-3-8-20(23(11-16)27(34)35)26-21-9-4-17(32)12-24(21)38-25-13-18(33)5-10-22(25)26/h1-13,32-33H,14H2,(H,30,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170763

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-{3-[4-(5-sul...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)Nc3ccc(c(c3)C(O)=O)-c3c4ccc(O)cc4oc4cc(=O)ccc34)cc2)s1 |(32.81,3.4,;31.27,3.4,;31.25,4.94,;31.25,1.86,;29.73,3.24,;28.96,1.89,;27.46,2.21,;27.3,3.73,;25.97,4.5,;24.62,3.71,;25.41,2.37,;23.83,5.06,;23.27,2.92,;21.94,3.69,;20.61,2.92,;20.61,1.38,;19.27,.61,;17.94,1.39,;17.95,2.93,;16.6,.62,;16.59,-.92,;15.24,-1.69,;15.24,-3.23,;16.59,-4,;17.92,-3.23,;17.92,-1.69,;19.26,-4.02,;20.59,-4.79,;20.59,-3.25,;16.59,-5.55,;15.26,-6.32,;13.93,-5.55,;12.58,-6.32,;12.58,-7.86,;11.25,-8.64,;13.93,-8.63,;15.26,-7.87,;16.59,-8.63,;17.93,-7.86,;19.26,-8.63,;20.59,-7.86,;21.94,-8.64,;20.59,-6.32,;19.26,-5.55,;17.93,-6.32,;21.94,.6,;23.27,1.37,;28.7,4.38,)| Show InChI InChI=1S/C29H20N6O9S4/c30-47(40,41)29-34-33-28(46-29)35-48(42,43)18-6-1-14(2-7-18)31-27(45)32-15-3-8-19(22(11-15)26(38)39)25-20-9-4-16(36)12-23(20)44-24-13-17(37)5-10-21(24)25/h1-13,36H,(H,33,35)(H,38,39)(H2,30,40,41)(H2,31,32,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170755
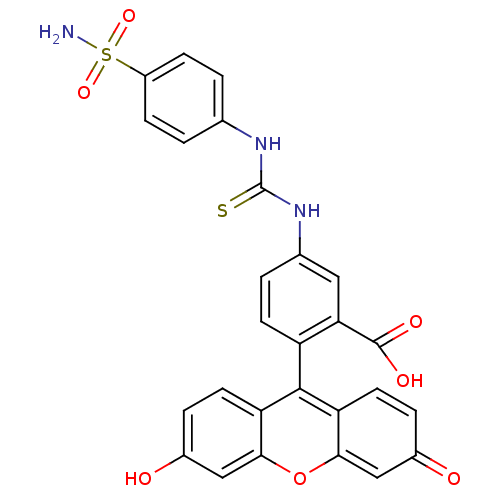
(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(4-sulfam...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)cc1 |(16.88,.93,;15.54,.16,;14.75,1.49,;16.32,-1.2,;14.19,-.64,;12.86,.13,;11.53,-.64,;11.53,-2.18,;10.2,-2.95,;8.87,-2.18,;8.87,-.64,;7.52,-2.94,;7.51,-4.48,;6.17,-5.25,;6.17,-6.8,;7.51,-7.57,;8.84,-6.8,;8.84,-5.25,;10.18,-7.58,;11.27,-8.66,;11.51,-6.81,;7.51,-9.11,;6.18,-9.88,;4.85,-9.11,;3.51,-9.88,;3.51,-11.42,;2.17,-12.21,;4.85,-12.19,;6.18,-11.43,;7.51,-12.19,;8.85,-11.42,;10.18,-12.19,;11.51,-11.42,;12.86,-12.21,;11.51,-9.88,;10.18,-9.12,;8.85,-9.88,;12.86,-2.95,;14.19,-2.18,)| Show InChI InChI=1S/C27H19N3O7S2/c28-39(35,36)18-6-1-14(2-7-18)29-27(38)30-15-3-8-19(22(11-15)26(33)34)25-20-9-4-16(31)12-23(20)37-24-13-17(32)5-10-21(24)25/h1-13,31H,(H,33,34)(H2,28,35,36)(H2,29,30,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170760
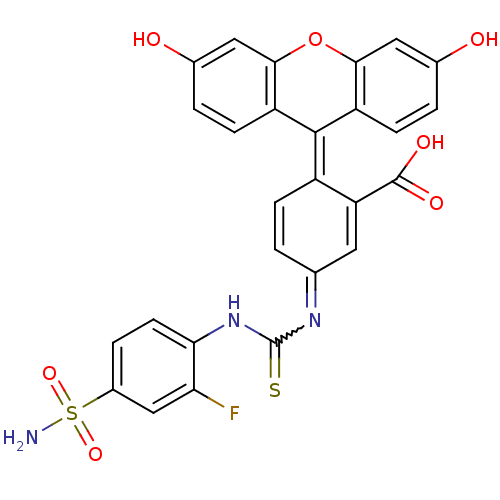
(5-[3-(2-Fluoro-4-sulfamoyl-phenyl)-thioureido]-2-(...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(F)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18FN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170757

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(2-iodo-4...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(I)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18IN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10890

(1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...)Show SMILES NS(=O)(=O)c1ccc(cc1)S(=O)(=O)Nc1cccc2c(Cl)c[nH]c12 Show InChI InChI=1S/C14H12ClN3O4S2/c15-12-8-17-14-11(12)2-1-3-13(14)18-24(21,22)10-6-4-9(5-7-10)23(16,19)20/h1-8,17-18H,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170761

(5-[3-(2-Chloro-4-sulfamoyl-phenyl)-thioureido]-2-(...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(Cl)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18ClN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10861
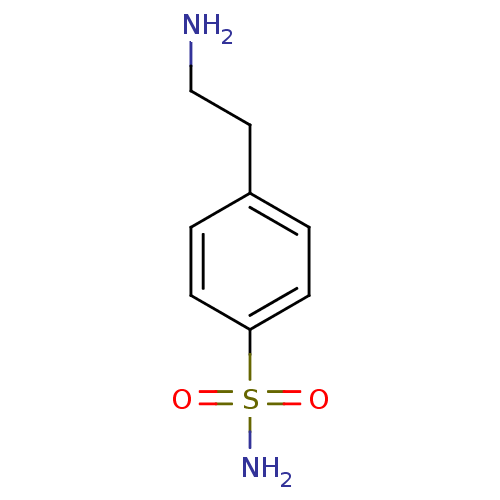
(4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...)Show InChI InChI=1S/C8H12N2O2S/c9-6-5-7-1-3-8(4-2-7)13(10,11)12/h1-4H,5-6,9H2,(H2,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170759

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(3-sulfam...)Show SMILES NS(=O)(=O)c1cccc(NC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c1 |(10.9,-.21,;9.55,.6,;8.76,-.75,;10.37,1.93,;8.22,1.37,;8.22,2.92,;6.89,3.69,;5.56,2.92,;5.55,1.38,;4.21,.61,;2.89,1.39,;2.9,2.93,;1.55,.62,;1.54,-.92,;.19,-1.69,;.19,-3.23,;1.54,-4,;2.87,-3.23,;2.87,-1.69,;4.21,-4.02,;5.54,-4.79,;5.54,-3.25,;1.54,-5.55,;.21,-6.32,;-1.12,-5.55,;-2.47,-6.32,;-2.47,-7.86,;-3.8,-8.64,;-1.12,-8.63,;.21,-7.87,;1.54,-8.63,;2.88,-7.86,;4.21,-8.63,;5.54,-7.86,;6.88,-8.64,;5.54,-6.32,;4.21,-5.55,;2.88,-6.32,;6.89,.6,)| Show InChI InChI=1S/C27H19N3O7S2/c28-39(35,36)18-3-1-2-14(10-18)29-27(38)30-15-4-7-19(22(11-15)26(33)34)25-20-8-5-16(31)12-23(20)37-24-13-17(32)6-9-21(24)25/h1-13,31H,(H,33,34)(H2,28,35,36)(H2,29,30,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170758

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-{3-[4-(4-sul...)Show SMILES NS(=O)(=O)c1ccc(CNS(=O)(=O)c2ccc(NC(=S)Nc3ccc(c(c3)C(O)=O)-c3c4ccc(O)cc4oc4cc(=O)ccc34)cc2)cc1 |(12.46,-6.66,;12.47,-5.12,;10.93,-5.11,;14.01,-5.12,;12.48,-3.58,;11.14,-2.81,;11.14,-1.27,;12.48,-.5,;12.47,1.04,;11.14,1.81,;9.8,1.02,;9.01,2.37,;10.58,-.33,;8.45,.23,;7.12,1,;5.79,.23,;5.79,-1.31,;4.44,-2.08,;3.11,-1.31,;3.13,.23,;1.78,-2.06,;1.76,-3.6,;.43,-4.37,;.43,-5.93,;1.76,-6.7,;3.09,-5.93,;3.09,-4.39,;4.43,-6.7,;5.76,-7.47,;5.76,-5.93,;1.76,-8.24,;.43,-9.01,;-.9,-8.24,;-2.24,-9.01,;-2.24,-10.55,;-3.59,-11.34,;-.9,-11.32,;.43,-10.55,;1.76,-11.32,;3.1,-10.55,;4.43,-11.32,;5.77,-10.55,;7.12,-11.34,;5.77,-9.01,;4.43,-8.24,;3.1,-9.01,;7.12,-2.08,;8.45,-1.31,;13.81,-1.26,;13.81,-2.8,)| Show InChI InChI=1S/C34H26N4O9S3/c35-49(43,44)24-8-1-19(2-9-24)18-36-50(45,46)25-10-3-20(4-11-25)37-34(48)38-21-5-12-26(29(15-21)33(41)42)32-27-13-6-22(39)16-30(27)47-31-17-23(40)7-14-28(31)32/h1-17,36,39H,18H2,(H,41,42)(H2,35,43,44)(H2,37,38,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170762

(5-[3-(2-Bromo-4-sulfamoyl-phenyl)-thioureido]-2-(6...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(Br)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18BrN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
MMDB
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50170753

(2,4,5-Trimethyl-1-(4-sulfamoyl-benzyl)-pyridinium;...)Show InChI InChI=1S/C15H19N2O2S/c1-11-8-13(3)17(9-12(11)2)10-14-4-6-15(7-5-14)20(16,18)19/h4-9H,10H2,1-3H3,(H2,16,18,19)/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170757

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(2-iodo-4...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(I)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18IN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170755

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(4-sulfam...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)cc1 |(16.88,.93,;15.54,.16,;14.75,1.49,;16.32,-1.2,;14.19,-.64,;12.86,.13,;11.53,-.64,;11.53,-2.18,;10.2,-2.95,;8.87,-2.18,;8.87,-.64,;7.52,-2.94,;7.51,-4.48,;6.17,-5.25,;6.17,-6.8,;7.51,-7.57,;8.84,-6.8,;8.84,-5.25,;10.18,-7.58,;11.27,-8.66,;11.51,-6.81,;7.51,-9.11,;6.18,-9.88,;4.85,-9.11,;3.51,-9.88,;3.51,-11.42,;2.17,-12.21,;4.85,-12.19,;6.18,-11.43,;7.51,-12.19,;8.85,-11.42,;10.18,-12.19,;11.51,-11.42,;12.86,-12.21,;11.51,-9.88,;10.18,-9.12,;8.85,-9.88,;12.86,-2.95,;14.19,-2.18,)| Show InChI InChI=1S/C27H19N3O7S2/c28-39(35,36)18-6-1-14(2-7-18)29-27(38)30-15-3-8-19(22(11-15)26(33)34)25-20-9-4-16(31)12-23(20)37-24-13-17(32)5-10-21(24)25/h1-13,31H,(H,33,34)(H2,28,35,36)(H2,29,30,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170762

(5-[3-(2-Bromo-4-sulfamoyl-phenyl)-thioureido]-2-(6...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(Br)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18BrN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170756

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(4-sulfam...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)cc1 |w:12.11,c:14,17| Show InChI InChI=1S/C28H21N3O7S2/c29-40(36,37)19-6-1-15(2-7-19)14-30-28(39)31-16-3-8-20(23(11-16)27(34)35)26-21-9-4-17(32)12-24(21)38-25-13-18(33)5-10-22(25)26/h1-13,32-33H,14H2,(H,30,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170754

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-{3-[2-(4-sul...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#6]-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)cc1 |w:13.12,c:15,18| Show InChI InChI=1S/C29H23N3O7S2/c30-41(37,38)20-6-1-16(2-7-20)11-12-31-29(40)32-17-3-8-21(24(13-17)28(35)36)27-22-9-4-18(33)14-25(22)39-26-15-19(34)5-10-23(26)27/h1-10,13-15,33-34H,11-12H2,(H,31,40)(H,35,36)(H2,30,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170760

(5-[3-(2-Fluoro-4-sulfamoyl-phenyl)-thioureido]-2-(...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(F)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18FN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human cloned isozyme (hCA IX) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170753

(2,4,5-Trimethyl-1-(4-sulfamoyl-benzyl)-pyridinium;...)Show InChI InChI=1S/C15H19N2O2S/c1-11-8-13(3)17(9-12(11)2)10-14-4-6-15(7-5-14)20(16,18)19/h4-9H,10H2,1-3H3,(H2,16,18,19)/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170761

(5-[3-(2-Chloro-4-sulfamoyl-phenyl)-thioureido]-2-(...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(Cl)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18ClN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50170759

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(3-sulfam...)Show SMILES NS(=O)(=O)c1cccc(NC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c1 |(10.9,-.21,;9.55,.6,;8.76,-.75,;10.37,1.93,;8.22,1.37,;8.22,2.92,;6.89,3.69,;5.56,2.92,;5.55,1.38,;4.21,.61,;2.89,1.39,;2.9,2.93,;1.55,.62,;1.54,-.92,;.19,-1.69,;.19,-3.23,;1.54,-4,;2.87,-3.23,;2.87,-1.69,;4.21,-4.02,;5.54,-4.79,;5.54,-3.25,;1.54,-5.55,;.21,-6.32,;-1.12,-5.55,;-2.47,-6.32,;-2.47,-7.86,;-3.8,-8.64,;-1.12,-8.63,;.21,-7.87,;1.54,-8.63,;2.88,-7.86,;4.21,-8.63,;5.54,-7.86,;6.88,-8.64,;5.54,-6.32,;4.21,-5.55,;2.88,-6.32,;6.89,.6,)| Show InChI InChI=1S/C27H19N3O7S2/c28-39(35,36)18-3-1-2-14(10-18)29-27(38)30-15-4-7-19(22(11-15)26(33)34)25-20-8-5-16(31)12-23(20)37-24-13-17(32)6-9-21(24)25/h1-13,31H,(H,33,34)(H2,28,35,36)(H2,29,30,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10861

(4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...)Show InChI InChI=1S/C8H12N2O2S/c9-6-5-7-1-3-8(4-2-7)13(10,11)12/h1-4H,5-6,9H2,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA II) by CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170763

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-{3-[4-(5-sul...)Show SMILES NS(=O)(=O)c1nnc(NS(=O)(=O)c2ccc(NC(=S)Nc3ccc(c(c3)C(O)=O)-c3c4ccc(O)cc4oc4cc(=O)ccc34)cc2)s1 |(32.81,3.4,;31.27,3.4,;31.25,4.94,;31.25,1.86,;29.73,3.24,;28.96,1.89,;27.46,2.21,;27.3,3.73,;25.97,4.5,;24.62,3.71,;25.41,2.37,;23.83,5.06,;23.27,2.92,;21.94,3.69,;20.61,2.92,;20.61,1.38,;19.27,.61,;17.94,1.39,;17.95,2.93,;16.6,.62,;16.59,-.92,;15.24,-1.69,;15.24,-3.23,;16.59,-4,;17.92,-3.23,;17.92,-1.69,;19.26,-4.02,;20.59,-4.79,;20.59,-3.25,;16.59,-5.55,;15.26,-6.32,;13.93,-5.55,;12.58,-6.32,;12.58,-7.86,;11.25,-8.64,;13.93,-8.63,;15.26,-7.87,;16.59,-8.63,;17.93,-7.86,;19.26,-8.63,;20.59,-7.86,;21.94,-8.64,;20.59,-6.32,;19.26,-5.55,;17.93,-6.32,;21.94,.6,;23.27,1.37,;28.7,4.38,)| Show InChI InChI=1S/C29H20N6O9S4/c30-47(40,41)29-34-33-28(46-29)35-48(42,43)18-6-1-14(2-7-18)31-27(45)32-15-3-8-19(22(11-15)26(38)39)25-20-9-4-16(36)12-23(20)44-24-13-17(37)5-10-21(24)25/h1-13,36H,(H,33,35)(H,38,39)(H2,30,40,41)(H2,31,32,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170758

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-{3-[4-(4-sul...)Show SMILES NS(=O)(=O)c1ccc(CNS(=O)(=O)c2ccc(NC(=S)Nc3ccc(c(c3)C(O)=O)-c3c4ccc(O)cc4oc4cc(=O)ccc34)cc2)cc1 |(12.46,-6.66,;12.47,-5.12,;10.93,-5.11,;14.01,-5.12,;12.48,-3.58,;11.14,-2.81,;11.14,-1.27,;12.48,-.5,;12.47,1.04,;11.14,1.81,;9.8,1.02,;9.01,2.37,;10.58,-.33,;8.45,.23,;7.12,1,;5.79,.23,;5.79,-1.31,;4.44,-2.08,;3.11,-1.31,;3.13,.23,;1.78,-2.06,;1.76,-3.6,;.43,-4.37,;.43,-5.93,;1.76,-6.7,;3.09,-5.93,;3.09,-4.39,;4.43,-6.7,;5.76,-7.47,;5.76,-5.93,;1.76,-8.24,;.43,-9.01,;-.9,-8.24,;-2.24,-9.01,;-2.24,-10.55,;-3.59,-11.34,;-.9,-11.32,;.43,-10.55,;1.76,-11.32,;3.1,-10.55,;4.43,-11.32,;5.77,-10.55,;7.12,-11.34,;5.77,-9.01,;4.43,-8.24,;3.1,-9.01,;7.12,-2.08,;8.45,-1.31,;13.81,-1.26,;13.81,-2.8,)| Show InChI InChI=1S/C34H26N4O9S3/c35-49(43,44)24-8-1-19(2-9-24)18-36-50(45,46)25-10-3-20(4-11-25)37-34(48)38-21-5-12-26(29(15-21)33(41)42)32-27-13-6-22(39)16-30(27)47-31-17-23(40)7-14-28(31)32/h1-17,36,39H,18H2,(H,41,42)(H2,35,43,44)(H2,37,38,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170761

(5-[3-(2-Chloro-4-sulfamoyl-phenyl)-thioureido]-2-(...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(Cl)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18ClN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170760

(5-[3-(2-Fluoro-4-sulfamoyl-phenyl)-thioureido]-2-(...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(F)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18FN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170757

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(2-iodo-4...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(I)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18IN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170762

(5-[3-(2-Bromo-4-sulfamoyl-phenyl)-thioureido]-2-(6...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)c(Br)c1 |w:11.10,c:13,16| Show InChI InChI=1S/C27H18BrN3O7S2/c28-21-12-16(40(29,36)37)4-8-22(21)31-27(39)30-13-1-5-17(20(9-13)26(34)35)25-18-6-2-14(32)10-23(18)38-24-11-15(33)3-7-19(24)25/h1-12,32-33H,(H,31,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10883

(4,5-dichlorobenzene-1,3-disulfonamide | CHEMBL17 |...)Show InChI InChI=1S/C6H6Cl2N2O4S2/c7-4-1-3(15(9,11)12)2-5(6(4)8)16(10,13)14/h1-2H,(H2,9,11,12)(H2,10,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170754

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-{3-[2-(4-sul...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#6]-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)cc1 |w:13.12,c:15,18| Show InChI InChI=1S/C29H23N3O7S2/c30-41(37,38)20-6-1-16(2-7-20)11-12-31-29(40)32-17-3-8-21(24(13-17)28(35)36)27-22-9-4-18(33)14-25(22)39-26-15-19(34)5-10-23(26)27/h1-10,13-15,33-34H,11-12H2,(H,31,40)(H,35,36)(H2,30,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170759

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(3-sulfam...)Show SMILES NS(=O)(=O)c1cccc(NC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)c1 |(10.9,-.21,;9.55,.6,;8.76,-.75,;10.37,1.93,;8.22,1.37,;8.22,2.92,;6.89,3.69,;5.56,2.92,;5.55,1.38,;4.21,.61,;2.89,1.39,;2.9,2.93,;1.55,.62,;1.54,-.92,;.19,-1.69,;.19,-3.23,;1.54,-4,;2.87,-3.23,;2.87,-1.69,;4.21,-4.02,;5.54,-4.79,;5.54,-3.25,;1.54,-5.55,;.21,-6.32,;-1.12,-5.55,;-2.47,-6.32,;-2.47,-7.86,;-3.8,-8.64,;-1.12,-8.63,;.21,-7.87,;1.54,-8.63,;2.88,-7.86,;4.21,-8.63,;5.54,-7.86,;6.88,-8.64,;5.54,-6.32,;4.21,-5.55,;2.88,-6.32,;6.89,.6,)| Show InChI InChI=1S/C27H19N3O7S2/c28-39(35,36)18-3-1-2-14(10-18)29-27(38)30-15-4-7-19(22(11-15)26(33)34)25-20-8-5-16(31)12-23(20)37-24-13-17(32)6-9-21(24)25/h1-13,31H,(H,33,34)(H2,28,35,36)(H2,29,30,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170756

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(4-sulfam...)Show SMILES [#7]S(=O)(=O)c1ccc(-[#6]-[#7]-[#6](=S)-[#7]=[#6]-2-[#6]=[#6]\[#6](-[#6](=[#6]-2)-[#6](-[#8])=O)=[#6]-2\c3ccc(-[#8])cc3-[#8]-c3cc(-[#8])ccc-23)cc1 |w:12.11,c:14,17| Show InChI InChI=1S/C28H21N3O7S2/c29-40(36,37)19-6-1-15(2-7-19)14-30-28(39)31-16-3-8-20(23(11-16)27(34)35)26-21-9-4-17(32)12-24(21)38-25-13-18(33)5-10-22(25)26/h1-13,32-33H,14H2,(H,30,39)(H,34,35)(H2,29,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170755

(2-(6-Hydroxy-3-oxo-3H-xanthen-9-yl)-5-[3-(4-sulfam...)Show SMILES NS(=O)(=O)c1ccc(NC(=S)Nc2ccc(c(c2)C(O)=O)-c2c3ccc(O)cc3oc3cc(=O)ccc23)cc1 |(16.88,.93,;15.54,.16,;14.75,1.49,;16.32,-1.2,;14.19,-.64,;12.86,.13,;11.53,-.64,;11.53,-2.18,;10.2,-2.95,;8.87,-2.18,;8.87,-.64,;7.52,-2.94,;7.51,-4.48,;6.17,-5.25,;6.17,-6.8,;7.51,-7.57,;8.84,-6.8,;8.84,-5.25,;10.18,-7.58,;11.27,-8.66,;11.51,-6.81,;7.51,-9.11,;6.18,-9.88,;4.85,-9.11,;3.51,-9.88,;3.51,-11.42,;2.17,-12.21,;4.85,-12.19,;6.18,-11.43,;7.51,-12.19,;8.85,-11.42,;10.18,-12.19,;11.51,-11.42,;12.86,-12.21,;11.51,-9.88,;10.18,-9.12,;8.85,-9.88,;12.86,-2.95,;14.19,-2.18,)| Show InChI InChI=1S/C27H19N3O7S2/c28-39(35,36)18-6-1-14(2-7-18)29-27(38)30-15-3-8-19(22(11-15)26(33)34)25-20-9-4-16(31)12-23(20)37-24-13-17(32)5-10-21(24)25/h1-13,31H,(H,33,34)(H2,28,35,36)(H2,29,30,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10861

(4-(2-aminoethyl)benzene-1-sulfonamide | CHEMBL7087...)Show InChI InChI=1S/C8H12N2O2S/c9-6-5-7-1-3-8(4-2-7)13(10,11)12/h1-4H,5-6,9H2,(H2,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50170753

(2,4,5-Trimethyl-1-(4-sulfamoyl-benzyl)-pyridinium;...)Show InChI InChI=1S/C15H19N2O2S/c1-11-8-13(3)17(9-12(11)2)10-14-4-6-15(7-5-14)20(16,18)19/h4-9H,10H2,1-3H3,(H2,16,18,19)/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition constant against human (cloned) isozyme (hCA I) by the CO2 hydration method |
J Med Chem 48: 4834-41 (2005)
Article DOI: 10.1021/jm0501073
BindingDB Entry DOI: 10.7270/Q21G0KS3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data