

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50171301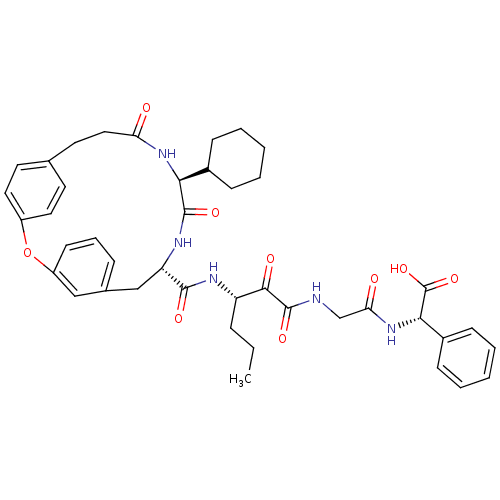 ((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50171302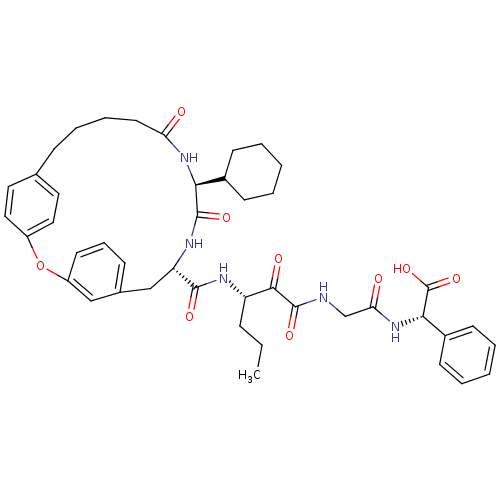 ((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50171302 ((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-chlorophenylbutyric acid ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV proteas... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50171305 ((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370596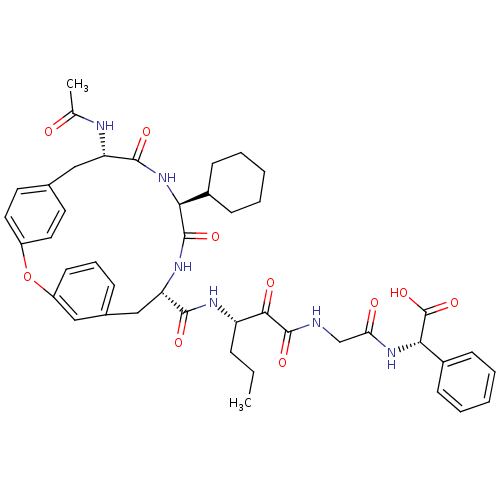 (CHEMBL1169404) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50171301 ((S)-(2-{3-[((9S,12S)-12-Cyclohexyl-11,14-dioxo-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Binding constant towards Human neutrophil elastase HNE protease | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50171304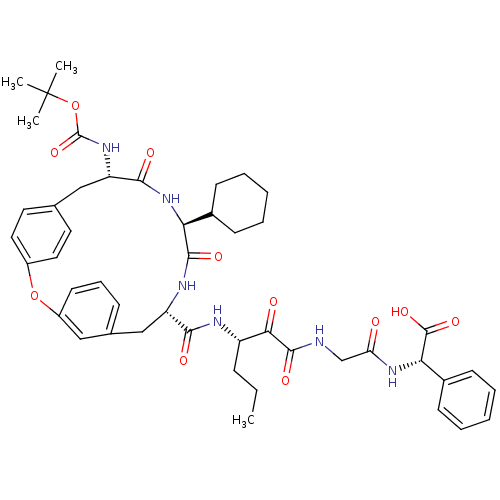 ((S)-(2-{(S)-3-[((9S,12S,15S)-15-tert-Butoxycarbony...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50171303 (CHEMBL370096 | {3-[((9S,12S,15S)-15-tert-Butoxycar...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Ability to inhibit the hydrolysis of chromogenic 4-phenylazophenyl ester from the peptide fragment Ac- DTEDVVP(Nva)-O-4-PAP in a HCV protease continu... | J Med Chem 48: 5088-91 (2005) Article DOI: 10.1021/jm0489556 BindingDB Entry DOI: 10.7270/Q2DN45T7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||