 Found 34 hits of Enzyme Inhibition Constant Data
Found 34 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM36609
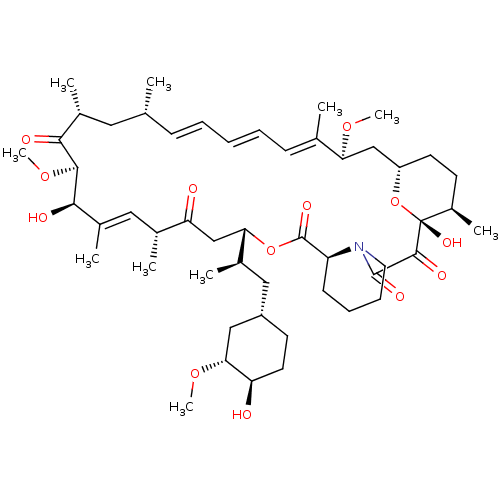
(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against FKBP12 receptor |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM36609

(Rapamycin C-7, analog 4 | SIROLIMUS | US11603377, ...)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12+,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38+,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding to FKBP12 |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50172395

((1R,9S,12R,21S,22S,24S,27R)-1-Hydroxy-22-methoxy-1...)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)N[C@H](CCc2ccccc2)C(=O)NCC(=O)N(C)CC(=O)O[C@H]1C Show InChI InChI=1S/C34H48N4O10/c1-21-13-15-24-18-27(46-4)22(2)47-29(40)20-37(3)28(39)19-35-31(42)25(16-14-23-10-6-5-7-11-23)36-32(43)26-12-8-9-17-38(26)33(44)30(41)34(21,45)48-24/h5-7,10-11,21-22,24-27,45H,8-9,12-20H2,1-4H3,(H,35,42)(H,36,43)/t21-,22+,24+,25-,26+,27+,34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against binding to FKBP12 |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50172395

((1R,9S,12R,21S,22S,24S,27R)-1-Hydroxy-22-methoxy-1...)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)N[C@H](CCc2ccccc2)C(=O)NCC(=O)N(C)CC(=O)O[C@H]1C Show InChI InChI=1S/C34H48N4O10/c1-21-13-15-24-18-27(46-4)22(2)47-29(40)20-37(3)28(39)19-35-31(42)25(16-14-23-10-6-5-7-11-23)36-32(43)26-12-8-9-17-38(26)33(44)30(41)34(21,45)48-24/h5-7,10-11,21-22,24-27,45H,8-9,12-20H2,1-4H3,(H,35,42)(H,36,43)/t21-,22+,24+,25-,26+,27+,34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against FKBP12 receptor |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50172384
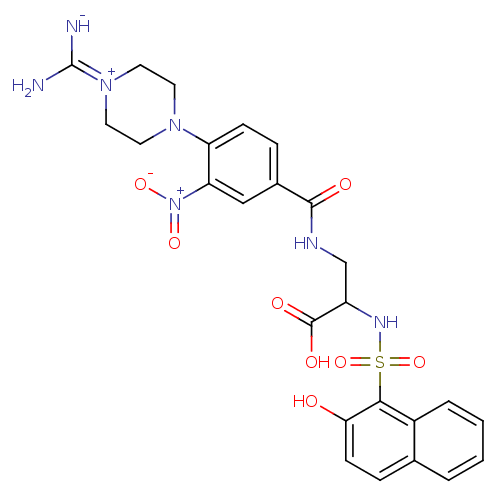
(3-[4-(4-Carbamimidoyl-piperazin-1-yl)-3-nitro-benz...)Show SMILES [#7]\[#6](-[#7-])=[#7+]-1\[#6]-[#6]-[#7](-[#6]-[#6]-1)-c1ccc(cc1-[#7+](-[#8-])=O)-[#6](=O)-[#7]-[#6]-[#6](-[#7]S(=O)(=O)c1c(-[#8])ccc2ccccc12)-[#6](-[#8])=O Show InChI InChI=1S/C25H27N7O8S/c26-25(27)31-11-9-30(10-12-31)19-7-5-16(13-20(19)32(37)38)23(34)28-14-18(24(35)36)29-41(39,40)22-17-4-2-1-3-15(17)6-8-21(22)33/h1-8,13,18,29H,9-12,14H2,(H6,26,27,28,33,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against alpha IIb beta-3 receptor |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50172384

(3-[4-(4-Carbamimidoyl-piperazin-1-yl)-3-nitro-benz...)Show SMILES [#7]\[#6](-[#7-])=[#7+]-1\[#6]-[#6]-[#7](-[#6]-[#6]-1)-c1ccc(cc1-[#7+](-[#8-])=O)-[#6](=O)-[#7]-[#6]-[#6](-[#7]S(=O)(=O)c1c(-[#8])ccc2ccccc12)-[#6](-[#8])=O Show InChI InChI=1S/C25H27N7O8S/c26-25(27)31-11-9-30(10-12-31)19-7-5-16(13-20(19)32(37)38)23(34)28-14-18(24(35)36)29-41(39,40)22-17-4-2-1-3-15(17)6-8-21(22)33/h1-8,13,18,29H,9-12,14H2,(H6,26,27,28,33,34,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against integrin alpha-2b beta3 |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Homo sapiens (Human)) | BDBM50172364
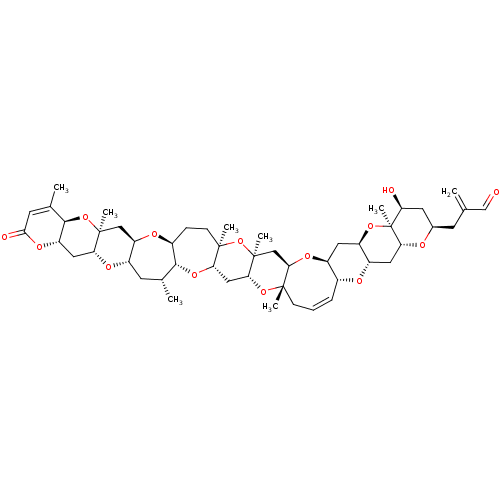
(2-{[(1R,3S,5R,7S,9R,11S,12S,14R,16R,18S,20R,21Z,24...)Show SMILES C[C@@H]1C[C@@H]2O[C@@H]3C[C@@H]4OC(=O)C=C(C)[C@H]4O[C@@]3(C)C[C@H]2O[C@H]2CC[C@@]3(C)O[C@@]4(C)C[C@H]5O[C@H]6C[C@H]7O[C@@]8(C)[C@@H](O)C[C@@H](CC(=C)C=O)O[C@@H]8C[C@@H]7O[C@@H]6\C=C/C[C@]5(C)O[C@@H]4C[C@@H]3O[C@H]12 |r,c:59,t:11| Show InChI InChI=1S/C50H70O14/c1-25(24-51)14-28-17-37(52)50(8)41(54-28)19-33-34(61-50)18-32-29(55-33)10-9-12-46(4)42(58-32)23-49(7)40(62-46)21-39-47(5,64-49)13-11-30-44(60-39)26(2)15-31-36(56-30)22-48(6)38(57-31)20-35-45(63-48)27(3)16-43(53)59-35/h9-10,16,24,26,28-42,44-45,52H,1,11-15,17-23H2,2-8H3/b10-9-/t26-,28-,29-,30+,31+,32+,33+,34-,35+,36-,37+,38-,39+,40-,41-,42-,44-,45-,46+,47-,48+,49+,50+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.0 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against synaptosome using [3H]PbTx-3 |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Homo sapiens (Human)) | BDBM50172364

(2-{[(1R,3S,5R,7S,9R,11S,12S,14R,16R,18S,20R,21Z,24...)Show SMILES C[C@@H]1C[C@@H]2O[C@@H]3C[C@@H]4OC(=O)C=C(C)[C@H]4O[C@@]3(C)C[C@H]2O[C@H]2CC[C@@]3(C)O[C@@]4(C)C[C@H]5O[C@H]6C[C@H]7O[C@@]8(C)[C@@H](O)C[C@@H](CC(=C)C=O)O[C@@H]8C[C@@H]7O[C@@H]6\C=C/C[C@]5(C)O[C@@H]4C[C@@H]3O[C@H]12 |r,c:59,t:11| Show InChI InChI=1S/C50H70O14/c1-25(24-51)14-28-17-37(52)50(8)41(54-28)19-33-34(61-50)18-32-29(55-33)10-9-12-46(4)42(58-32)23-49(7)40(62-46)21-39-47(5,64-49)13-11-30-44(60-39)26(2)15-31-36(56-30)22-48(6)38(57-31)20-35-45(63-48)27(3)16-43(53)59-35/h9-10,16,24,26,28-42,44-45,52H,1,11-15,17-23H2,2-8H3/b10-9-/t26-,28-,29-,30+,31+,32+,33+,34-,35+,36-,37+,38-,39+,40-,41-,42-,44-,45-,46+,47-,48+,49+,50+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.0 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against synaptosomes using [3H]PbTx-3 |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit NDUFA4
(Homo sapiens (Human)) | BDBM50172374
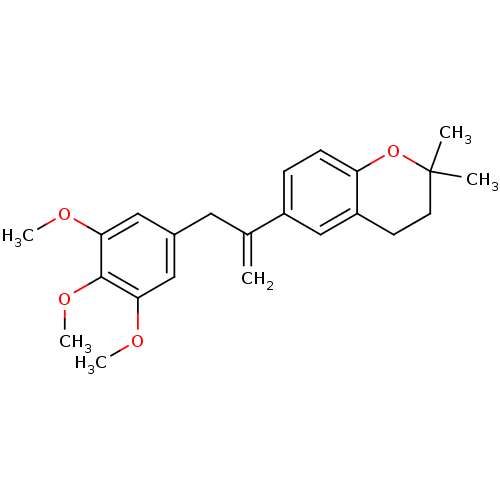
(2,2-Dimethyl-6-[1-(3,4,5-trimethoxy-benzyl)-vinyl]...)Show SMILES COc1cc(CC(=C)c2ccc3OC(C)(C)CCc3c2)cc(OC)c1OC Show InChI InChI=1S/C23H28O4/c1-15(11-16-12-20(24-4)22(26-6)21(13-16)25-5)17-7-8-19-18(14-17)9-10-23(2,3)27-19/h7-8,12-14H,1,9-11H2,2-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against NADH-ubiquinone oxidoreductase |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit NDUFA4
(Homo sapiens (Human)) | BDBM50172374

(2,2-Dimethyl-6-[1-(3,4,5-trimethoxy-benzyl)-vinyl]...)Show SMILES COc1cc(CC(=C)c2ccc3OC(C)(C)CCc3c2)cc(OC)c1OC Show InChI InChI=1S/C23H28O4/c1-15(11-16-12-20(24-4)22(26-6)21(13-16)25-5)17-7-8-19-18(14-17)9-10-23(2,3)27-19/h7-8,12-14H,1,9-11H2,2-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against NADH ubiquinone oxidoreductase |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit NDUFA4
(Homo sapiens (Human)) | BDBM50172381
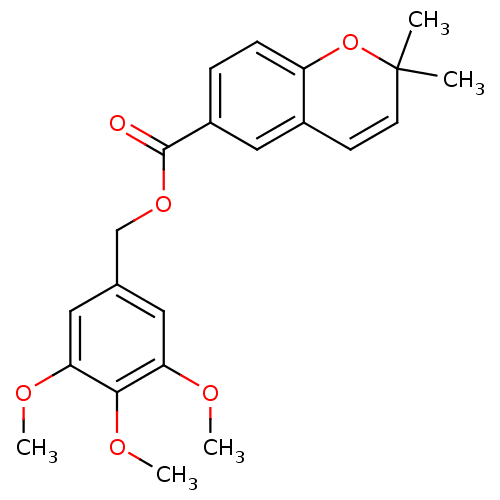
(2,2-Dimethyl-2H-chromene-6-carboxylic acid 3,4,5-t...)Show SMILES COc1cc(COC(=O)c2ccc3OC(C)(C)C=Cc3c2)cc(OC)c1OC |c:17| Show InChI InChI=1S/C22H24O6/c1-22(2)9-8-15-12-16(6-7-17(15)28-22)21(23)27-13-14-10-18(24-3)20(26-5)19(11-14)25-4/h6-12H,13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against NADH-ubiquinone oxidoreductase |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Cytochrome c oxidase subunit NDUFA4
(Homo sapiens (Human)) | BDBM50172381

(2,2-Dimethyl-2H-chromene-6-carboxylic acid 3,4,5-t...)Show SMILES COc1cc(COC(=O)c2ccc3OC(C)(C)C=Cc3c2)cc(OC)c1OC |c:17| Show InChI InChI=1S/C22H24O6/c1-22(2)9-8-15-12-16(6-7-17(15)28-22)21(23)27-13-14-10-18(24-3)20(26-5)19(11-14)25-4/h6-12H,13H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against NADH ubiquinone oxidoreductase |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Mycothiol S-conjugate amidase
(Mycobacterium tuberculosis) | BDBM50117090
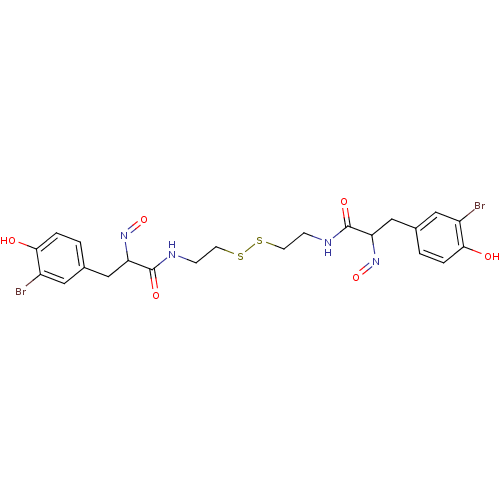
((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...)Show SMILES Oc1ccc(CC(N=O)C(=O)NCCSSCCNC(=O)C(Cc2ccc(O)c(Br)c2)N=O)cc1Br Show InChI InChI=1S/C22H24Br2N4O6S2/c23-15-9-13(1-3-19(15)29)11-17(27-33)21(31)25-5-7-35-36-8-6-26-22(32)18(28-34)12-14-2-4-20(30)16(24)10-14/h1-4,9-10,17-18,29-30H,5-8,11-12H2,(H,25,31)(H,26,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Mycothiol S-conjugate amidase
(Mycobacterium tuberculosis) | BDBM50117090

((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...)Show SMILES Oc1ccc(CC(N=O)C(=O)NCCSSCCNC(=O)C(Cc2ccc(O)c(Br)c2)N=O)cc1Br Show InChI InChI=1S/C22H24Br2N4O6S2/c23-15-9-13(1-3-19(15)29)11-17(27-33)21(31)25-5-7-35-36-8-6-26-22(32)18(28-34)12-14-2-4-20(30)16(24)10-14/h1-4,9-10,17-18,29-30H,5-8,11-12H2,(H,25,31)(H,26,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Homo sapiens (Human)) | BDBM50172380

(CHEMBL370148 | Truncated brevetoxin B)Show SMILES CC1=CC(=O)O[C@H]2C[C@H]3O[C@@]4(C)C\C=C/[C@H]5O[C@H]6C[C@H]7O[C@H](CC(=C)C=O)C[C@H](O)[C@]7(C)O[C@@H]6C[C@@H]5O[C@@H]4C[C@]3(C)O[C@]12C |c:13,t:1| Show InChI InChI=1S/C34H46O10/c1-18(17-35)10-20-12-25(36)34(6)27(38-20)14-23-24(42-34)13-22-21(39-23)8-7-9-31(3)29(40-22)16-32(4)26(43-31)15-28-33(5,44-32)19(2)11-30(37)41-28/h7-8,11,17,20-29,36H,1,9-10,12-16H2,2-6H3/b8-7-/t20-,21-,22+,23+,24-,25+,26-,27-,28+,29-,31+,32+,33-,34+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against synaptosome using [3H]PbTx-3 |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 1/2/3 subunit alpha
(Homo sapiens (Human)) | BDBM50172380

(CHEMBL370148 | Truncated brevetoxin B)Show SMILES CC1=CC(=O)O[C@H]2C[C@H]3O[C@@]4(C)C\C=C/[C@H]5O[C@H]6C[C@H]7O[C@H](CC(=C)C=O)C[C@H](O)[C@]7(C)O[C@@H]6C[C@@H]5O[C@@H]4C[C@]3(C)O[C@]12C |c:13,t:1| Show InChI InChI=1S/C34H46O10/c1-18(17-35)10-20-12-25(36)34(6)27(38-20)14-23-24(42-34)13-22-21(39-23)8-7-9-31(3)29(40-22)16-32(4)26(43-31)15-28-33(5,44-32)19(2)11-30(37)41-28/h7-8,11,17,20-29,36H,1,9-10,12-16H2,2-6H3/b8-7-/t20-,21-,22+,23+,24-,25+,26-,27-,28+,29-,31+,32+,33-,34+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory concentration against synaptosomes using [3H]PbTx-3 |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Mycothiol S-conjugate amidase
(Mycobacterium tuberculosis) | BDBM50117078
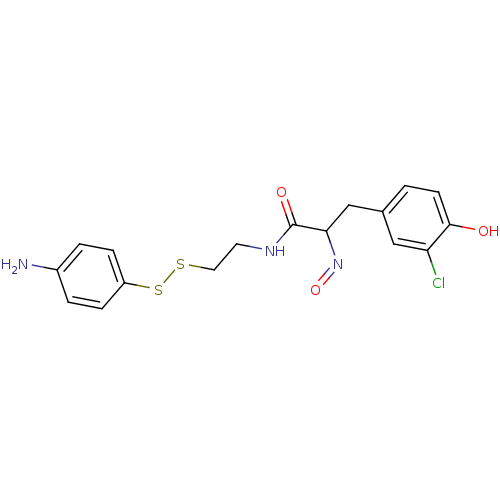
(CHEMBL197081 | CHEMBL312123 | N-[2-(4-Amino-phenyl...)Show SMILES Nc1ccc(SSCCNC(=O)C(Cc2ccc(O)c(Cl)c2)N=O)cc1 Show InChI InChI=1S/C17H18ClN3O3S2/c18-14-9-11(1-6-16(14)22)10-15(21-24)17(23)20-7-8-25-26-13-4-2-12(19)3-5-13/h1-6,9,15,22H,7-8,10,19H2,(H,20,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Mycothiol S-conjugate amidase
(Mycobacterium tuberculosis) | BDBM50117078

(CHEMBL197081 | CHEMBL312123 | N-[2-(4-Amino-phenyl...)Show SMILES Nc1ccc(SSCCNC(=O)C(Cc2ccc(O)c(Cl)c2)N=O)cc1 Show InChI InChI=1S/C17H18ClN3O3S2/c18-14-9-11(1-6-16(14)22)10-15(21-24)17(23)20-7-8-25-26-13-4-2-12(19)3-5-13/h1-6,9,15,22H,7-8,10,19H2,(H,20,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Mycothiol S-conjugate amidase
(Mycobacterium tuberculosis) | BDBM50117083
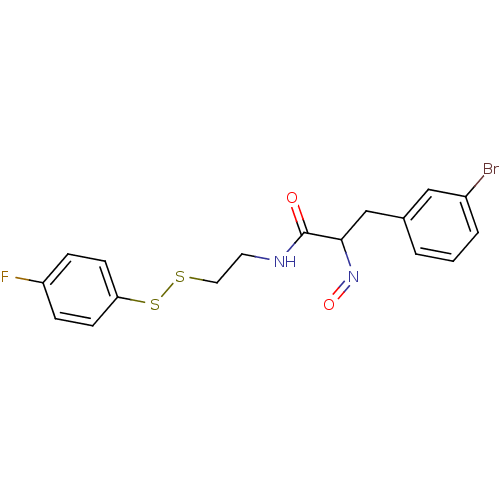
(3-(3-Bromo-phenyl)-N-[2-(4-fluoro-phenyldisulfanyl...)Show InChI InChI=1S/C17H16BrFN2O2S2/c18-13-3-1-2-12(10-13)11-16(21-23)17(22)20-8-9-24-25-15-6-4-14(19)5-7-15/h1-7,10,16H,8-9,11H2,(H,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Mycothiol S-conjugate amidase
(Mycobacterium tuberculosis) | BDBM50117083

(3-(3-Bromo-phenyl)-N-[2-(4-fluoro-phenyldisulfanyl...)Show InChI InChI=1S/C17H16BrFN2O2S2/c18-13-3-1-2-12(10-13)11-16(21-23)17(22)20-8-9-24-25-15-6-4-14(19)5-7-15/h1-7,10,16H,8-9,11H2,(H,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Mycothiol S-conjugate amidase
(Mycobacterium tuberculosis) | BDBM50117087
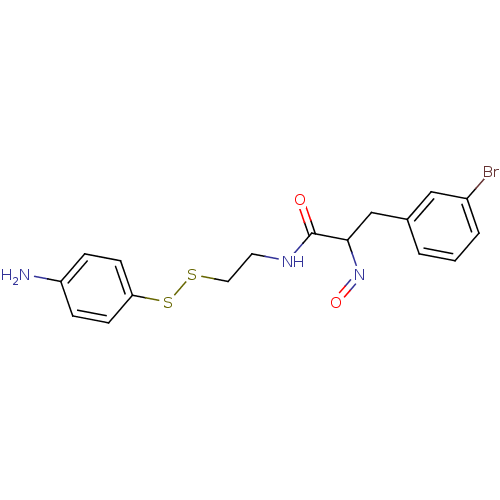
(CHEMBL194868 | CHEMBL312219 | N-[2-(4-Amino-phenyl...)Show InChI InChI=1S/C17H18BrN3O2S2/c18-13-3-1-2-12(10-13)11-16(21-23)17(22)20-8-9-24-25-15-6-4-14(19)5-7-15/h1-7,10,16H,8-9,11,19H2,(H,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Mycothiol S-conjugate amidase
(Mycobacterium tuberculosis) | BDBM50117087

(CHEMBL194868 | CHEMBL312219 | N-[2-(4-Amino-phenyl...)Show InChI InChI=1S/C17H18BrN3O2S2/c18-13-3-1-2-12(10-13)11-16(21-23)17(22)20-8-9-24-25-15-6-4-14(19)5-7-15/h1-7,10,16H,8-9,11,19H2,(H,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Mycothiol S-conjugate amidase
(Mycobacterium tuberculosis) | BDBM50117080
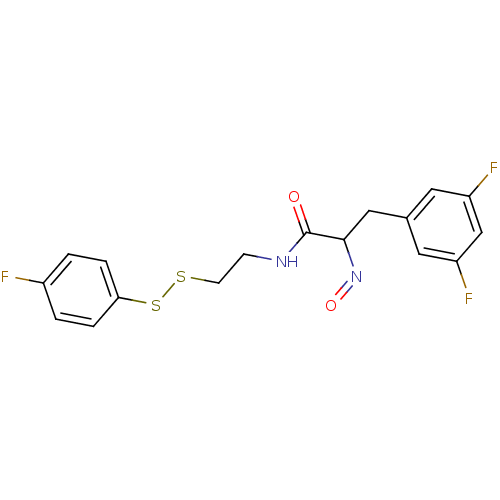
(3-(3,5-Difluoro-phenyl)-N-[2-(4-fluoro-phenyldisul...)Show InChI InChI=1S/C17H15F3N2O2S2/c18-12-1-3-15(4-2-12)26-25-6-5-21-17(23)16(22-24)9-11-7-13(19)10-14(20)8-11/h1-4,7-8,10,16H,5-6,9H2,(H,21,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.72E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Mycothiol S-conjugate amidase
(Mycobacterium tuberculosis) | BDBM50117080

(3-(3,5-Difluoro-phenyl)-N-[2-(4-fluoro-phenyldisul...)Show InChI InChI=1S/C17H15F3N2O2S2/c18-12-1-3-15(4-2-12)26-25-6-5-21-17(23)16(22-24)9-11-7-13(19)10-14(20)8-11/h1-4,7-8,10,16H,5-6,9H2,(H,21,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.72E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis detoxification enzyme mycothiol-S-conjugate amidase (MCA) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50167161
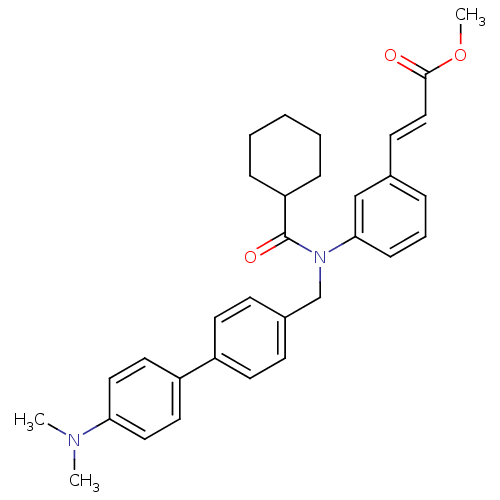
((E)-3-{3-[Cyclohexanecarbonyl-(4'-dimethylamino-bi...)Show SMILES COC(=O)\C=C\c1cccc(c1)N(Cc1ccc(cc1)-c1ccc(cc1)N(C)C)C(=O)C1CCCCC1 Show InChI InChI=1S/C32H36N2O3/c1-33(2)29-19-17-27(18-20-29)26-15-12-25(13-16-26)23-34(32(36)28-9-5-4-6-10-28)30-11-7-8-24(22-30)14-21-31(35)37-3/h7-8,11-22,28H,4-6,9-10,23H2,1-3H3/b21-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration against farnesoid X receptor (FXR) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50172379
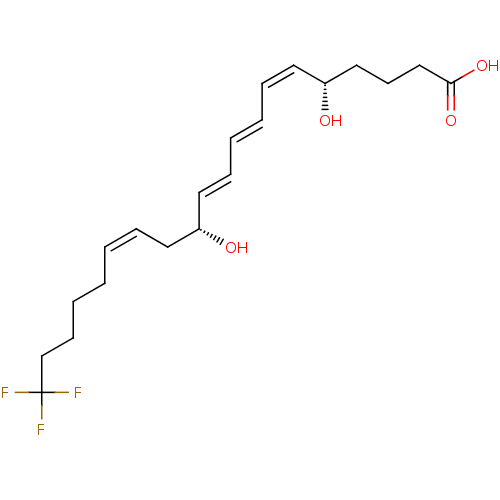
((8E,10E)-(5S,9R)-20,20,20-Trifluoro-5,12-dihydroxy...)Show SMILES O[C@@H](CCCC(O)=O)\C=C/C=C/C=C/[C@H](O)C\C=C/CCCCC(F)(F)F Show InChI InChI=1S/C20H29F3O4/c21-20(22,23)16-9-5-1-2-6-11-17(24)12-7-3-4-8-13-18(25)14-10-15-19(26)27/h2-4,6-8,12-13,17-18,24-25H,1,5,9-11,14-16H2,(H,26,27)/b4-3+,6-2-,12-7+,13-8-/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration required to inhibit 5-lipoxygenase by 50% |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50172379

((8E,10E)-(5S,9R)-20,20,20-Trifluoro-5,12-dihydroxy...)Show SMILES O[C@@H](CCCC(O)=O)\C=C/C=C/C=C/[C@H](O)C\C=C/CCCCC(F)(F)F Show InChI InChI=1S/C20H29F3O4/c21-20(22,23)16-9-5-1-2-6-11-17(24)12-7-3-4-8-13-18(25)14-10-15-19(26)27/h2-4,6-8,12-13,17-18,24-25H,1,5,9-11,14-16H2,(H,26,27)/b4-3+,6-2-,12-7+,13-8-/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration required to inhibit 5-lipoxygenase by 50% |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50172340
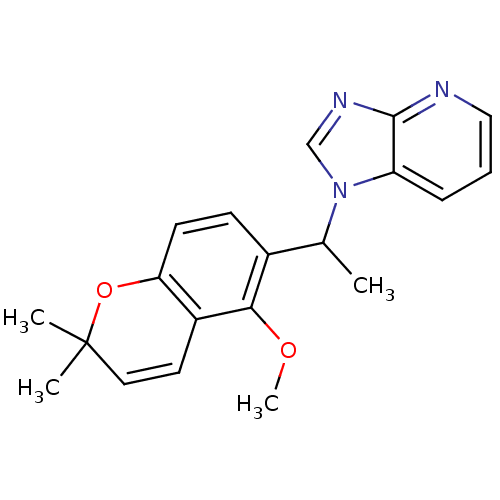
(1-[1-(5-Methoxy-2,2-dimethyl-2H-chromen-6-yl)-ethy...)Show SMILES COc1c(ccc2OC(C)(C)C=Cc12)C(C)n1cnc2ncccc12 |c:11| Show InChI InChI=1S/C20H21N3O2/c1-13(23-12-22-19-16(23)6-5-11-21-19)14-7-8-17-15(18(14)24-4)9-10-20(2,3)25-17/h5-13H,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration to inhibit production of hypoxia inducible factor 1-alpha protein |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50172373
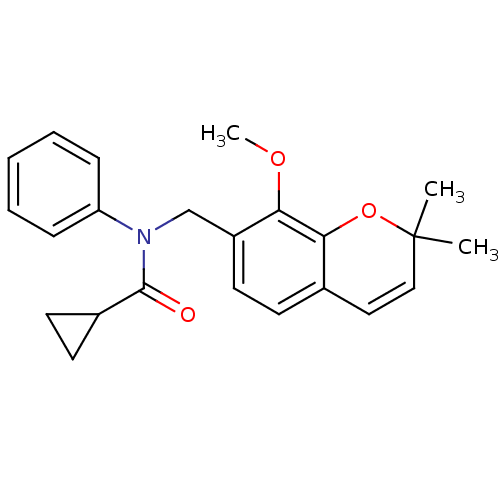
(CHEMBL197473 | Cyclopropanecarboxylic acid (8-meth...)Show SMILES COc1c(CN(C(=O)C2CC2)c2ccccc2)ccc2C=CC(C)(C)Oc12 |c:22| Show InChI InChI=1S/C23H25NO3/c1-23(2)14-13-16-9-12-18(20(26-3)21(16)27-23)15-24(22(25)17-10-11-17)19-7-5-4-6-8-19/h4-9,12-14,17H,10-11,15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration against farnesoid X receptor (FXR); range is 5-10 uM |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50172373

(CHEMBL197473 | Cyclopropanecarboxylic acid (8-meth...)Show SMILES COc1c(CN(C(=O)C2CC2)c2ccccc2)ccc2C=CC(C)(C)Oc12 |c:22| Show InChI InChI=1S/C23H25NO3/c1-23(2)14-13-16-9-12-18(20(26-3)21(16)27-23)15-24(22(25)17-10-11-17)19-7-5-4-6-8-19/h4-9,12-14,17H,10-11,15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration towards farnesoid X receptor (FXR); Range is 5-10 uM |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50172358
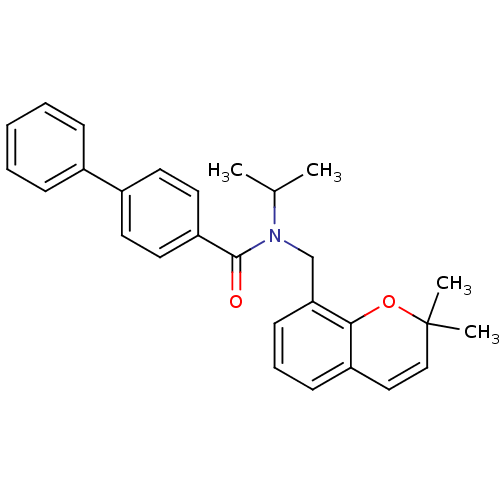
(Biphenyl-4-carboxylic acid (2,2-dimethyl-2H-chrome...)Show SMILES CC(C)N(Cc1cccc2C=CC(C)(C)Oc12)C(=O)c1ccc(cc1)-c1ccccc1 |c:10| Show InChI InChI=1S/C28H29NO2/c1-20(2)29(19-25-12-8-11-23-17-18-28(3,4)31-26(23)25)27(30)24-15-13-22(14-16-24)21-9-6-5-7-10-21/h5-18,20H,19H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration against PPAR delta receptor |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50172340

(1-[1-(5-Methoxy-2,2-dimethyl-2H-chromen-6-yl)-ethy...)Show SMILES COc1c(ccc2OC(C)(C)C=Cc12)C(C)n1cnc2ncccc12 |c:11| Show InChI InChI=1S/C20H21N3O2/c1-13(23-12-22-19-16(23)6-5-11-21-19)14-7-8-17-15(18(14)24-4)9-10-20(2,3)25-17/h5-13H,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration to inhibit production of hypoxia inducible factor 1-alpha protein |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50172358

(Biphenyl-4-carboxylic acid (2,2-dimethyl-2H-chrome...)Show SMILES CC(C)N(Cc1cccc2C=CC(C)(C)Oc12)C(=O)c1ccc(cc1)-c1ccccc1 |c:10| Show InChI InChI=1S/C28H29NO2/c1-20(2)29(19-25-12-8-11-23-17-18-28(3,4)31-26(23)25)27(30)24-15-13-22(14-16-24)21-9-6-5-7-10-21/h5-18,20H,19H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration towards PPAR delta |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50167161

((E)-3-{3-[Cyclohexanecarbonyl-(4'-dimethylamino-bi...)Show SMILES COC(=O)\C=C\c1cccc(c1)N(Cc1ccc(cc1)-c1ccc(cc1)N(C)C)C(=O)C1CCCCC1 Show InChI InChI=1S/C32H36N2O3/c1-33(2)29-19-17-27(18-20-29)26-15-12-25(13-16-26)23-34(32(36)28-9-5-4-6-10-28)30-11-7-8-24(22-30)14-21-31(35)37-3/h7-8,11-22,28H,4-6,9-10,23H2,1-3H3/b21-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| MMDB
Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Effective concentration towards farnesoid X receptor (FXR) |
J Med Chem 48: 5613-38 (2005)
Article DOI: 10.1021/jm050524f
BindingDB Entry DOI: 10.7270/Q2V988V1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data