 Found 14 hits of Enzyme Inhibition Constant Data
Found 14 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50173167
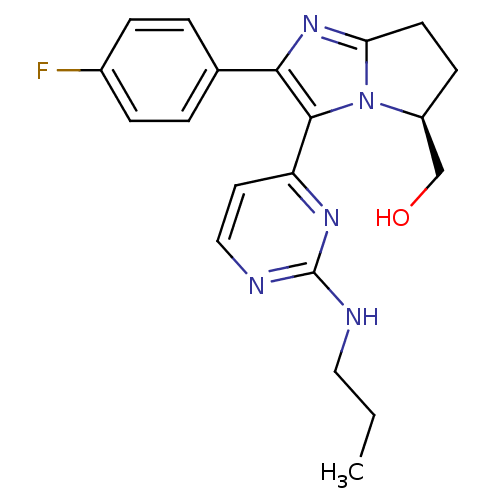
((S)-(2-(4-fluorophenyl)-3-(2-(propylamino)pyrimidi...)Show SMILES CCCNc1nccc(n1)-c1c(nc2CC[C@@H](CO)n12)-c1ccc(F)cc1 Show InChI InChI=1S/C20H22FN5O/c1-2-10-22-20-23-11-9-16(24-20)19-18(13-3-5-14(21)6-4-13)25-17-8-7-15(12-27)26(17)19/h3-6,9,11,15,27H,2,7-8,10,12H2,1H3,(H,22,23,24)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50173169
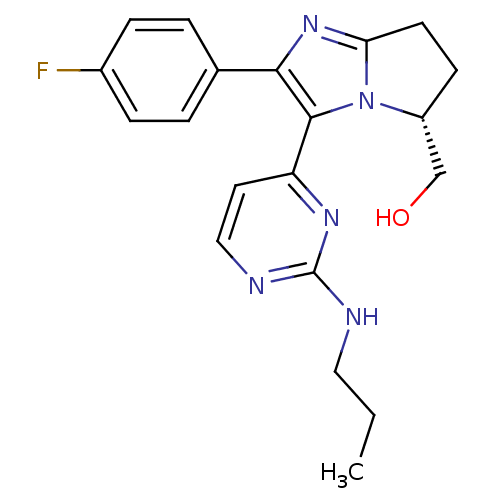
(CHEMBL383799 | [(R)-2-(4-Fluoro-phenyl)-3-(2-propy...)Show SMILES CCCNc1nccc(n1)-c1c(nc2CC[C@H](CO)n12)-c1ccc(F)cc1 Show InChI InChI=1S/C20H22FN5O/c1-2-10-22-20-23-11-9-16(24-20)19-18(13-3-5-14(21)6-4-13)25-17-8-7-15(12-27)26(17)19/h3-6,9,11,15,27H,2,7-8,10,12H2,1H3,(H,22,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50173173

(CHEMBL383348 | {4-[(S)-2-(4-Fluoro-phenyl)-5-(4-me...)Show SMILES CCCNc1nccc(n1)-c1c(nc2CC[C@@H](COCc3ccc(OC)cc3)n12)-c1ccc(F)cc1 Show InChI InChI=1S/C28H30FN5O2/c1-3-15-30-28-31-16-14-24(32-28)27-26(20-6-8-21(29)9-7-20)33-25-13-10-22(34(25)27)18-36-17-19-4-11-23(35-2)12-5-19/h4-9,11-12,14,16,22H,3,10,13,15,17-18H2,1-2H3,(H,30,31,32)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50173167

((S)-(2-(4-fluorophenyl)-3-(2-(propylamino)pyrimidi...)Show SMILES CCCNc1nccc(n1)-c1c(nc2CC[C@@H](CO)n12)-c1ccc(F)cc1 Show InChI InChI=1S/C20H22FN5O/c1-2-10-22-20-23-11-9-16(24-20)19-18(13-3-5-14(21)6-4-13)25-17-8-7-15(12-27)26(17)19/h3-6,9,11,15,27H,2,7-8,10,12H2,1H3,(H,22,23,24)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50173169

(CHEMBL383799 | [(R)-2-(4-Fluoro-phenyl)-3-(2-propy...)Show SMILES CCCNc1nccc(n1)-c1c(nc2CC[C@H](CO)n12)-c1ccc(F)cc1 Show InChI InChI=1S/C20H22FN5O/c1-2-10-22-20-23-11-9-16(24-20)19-18(13-3-5-14(21)6-4-13)25-17-8-7-15(12-27)26(17)19/h3-6,9,11,15,27H,2,7-8,10,12H2,1H3,(H,22,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50173166
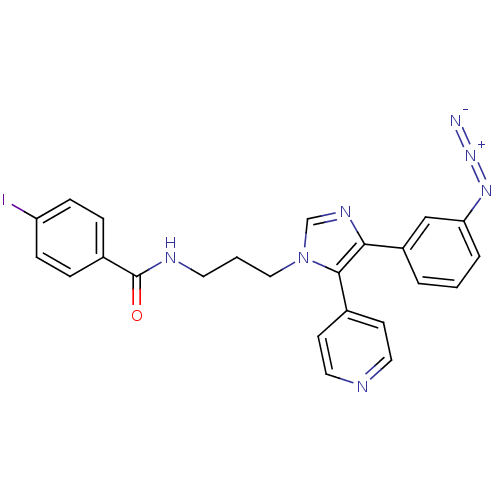
(CHEMBL371720 | N-{3-[4-(3-Azido-phenyl)-5-pyridin-...)Show SMILES Ic1ccc(cc1)C(=O)NCCCn1cnc(c1-c1ccncc1)-c1cccc(c1)N=[N+]=[N-] Show InChI InChI=1S/C24H20IN7O/c25-20-7-5-18(6-8-20)24(33)28-11-2-14-32-16-29-22(23(32)17-9-12-27-13-10-17)19-3-1-4-21(15-19)30-31-26/h1,3-10,12-13,15-16H,2,11,14H2,(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory concentration against p38 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50173173

(CHEMBL383348 | {4-[(S)-2-(4-Fluoro-phenyl)-5-(4-me...)Show SMILES CCCNc1nccc(n1)-c1c(nc2CC[C@@H](COCc3ccc(OC)cc3)n12)-c1ccc(F)cc1 Show InChI InChI=1S/C28H30FN5O2/c1-3-15-30-28-31-16-14-24(32-28)27-26(20-6-8-21(29)9-7-20)33-25-13-10-22(34(25)27)18-36-17-19-4-11-23(35-2)12-5-19/h4-9,11-12,14,16,22H,3,10,13,15,17-18H2,1-2H3,(H,30,31,32)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50173168

(4-[(S)-2-(4-Fluoro-phenyl)-5-(4-methoxy-benzyloxym...)Show SMILES COc1ccc(COC[C@@H]2CCc3nc(c(-c4ccnc(N)n4)n23)-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C25H24FN5O2/c1-32-20-9-2-16(3-10-20)14-33-15-19-8-11-22-30-23(17-4-6-18(26)7-5-17)24(31(19)22)21-12-13-28-25(27)29-21/h2-7,9-10,12-13,19H,8,11,14-15H2,1H3,(H2,27,28,29)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61.2 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50173172
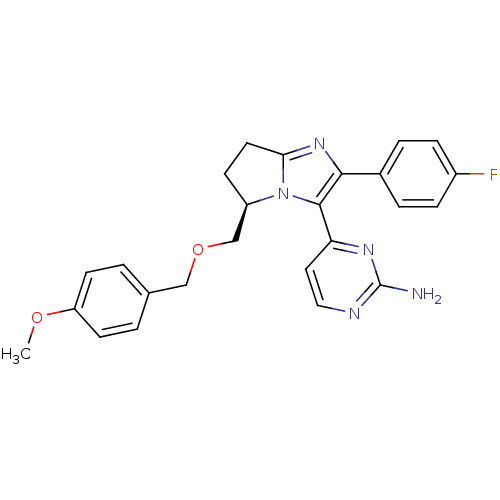
(4-[(R)-2-(4-Fluoro-phenyl)-5-(4-methoxy-benzyloxym...)Show SMILES COc1ccc(COC[C@H]2CCc3nc(c(-c4ccnc(N)n4)n23)-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C25H24FN5O2/c1-32-20-9-2-16(3-10-20)14-33-15-19-8-11-22-30-23(17-4-6-18(26)7-5-17)24(31(19)22)21-12-13-28-25(27)29-21/h2-7,9-10,12-13,19H,8,11,14-15H2,1H3,(H2,27,28,29)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50173171
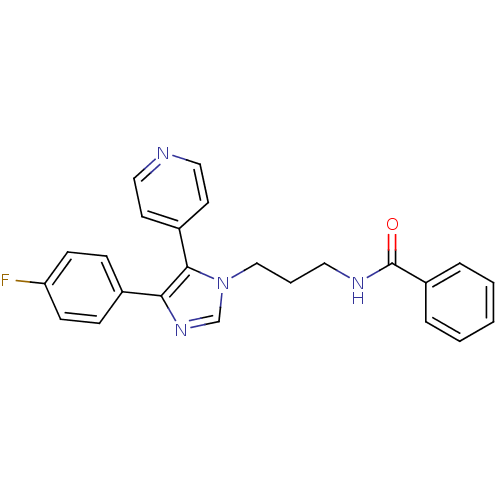
(CHEMBL199247 | N-(3-(4-(4-fluorophenyl)-5-(pyridin...)Show SMILES Fc1ccc(cc1)-c1ncn(CCCNC(=O)c2ccccc2)c1-c1ccncc1 Show InChI InChI=1S/C24H21FN4O/c25-21-9-7-18(8-10-21)22-23(19-11-14-26-15-12-19)29(17-28-22)16-4-13-27-24(30)20-5-2-1-3-6-20/h1-3,5-12,14-15,17H,4,13,16H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory concentration against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50173170
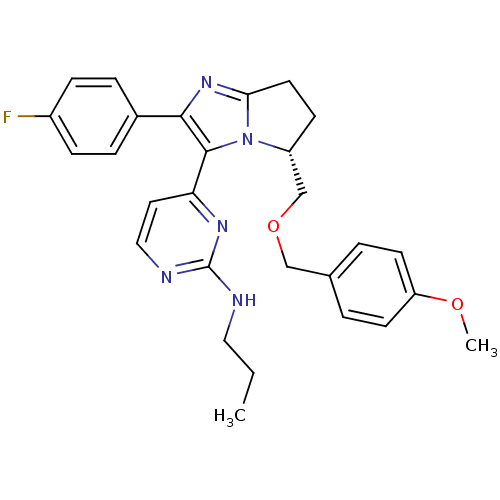
(CHEMBL198261 | {4-[(R)-2-(4-Fluoro-phenyl)-5-(4-me...)Show SMILES CCCNc1nccc(n1)-c1c(nc2CC[C@H](COCc3ccc(OC)cc3)n12)-c1ccc(F)cc1 Show InChI InChI=1S/C28H30FN5O2/c1-3-15-30-28-31-16-14-24(32-28)27-26(20-6-8-21(29)9-7-20)33-25-13-10-22(34(25)27)18-36-17-19-4-11-23(35-2)12-5-19/h4-9,11-12,14,16,22H,3,10,13,15,17-18H2,1-2H3,(H,30,31,32)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 217 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50173170

(CHEMBL198261 | {4-[(R)-2-(4-Fluoro-phenyl)-5-(4-me...)Show SMILES CCCNc1nccc(n1)-c1c(nc2CC[C@H](COCc3ccc(OC)cc3)n12)-c1ccc(F)cc1 Show InChI InChI=1S/C28H30FN5O2/c1-3-15-30-28-31-16-14-24(32-28)27-26(20-6-8-21(29)9-7-20)33-25-13-10-22(34(25)27)18-36-17-19-4-11-23(35-2)12-5-19/h4-9,11-12,14,16,22H,3,10,13,15,17-18H2,1-2H3,(H,30,31,32)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50173172

(4-[(R)-2-(4-Fluoro-phenyl)-5-(4-methoxy-benzyloxym...)Show SMILES COc1ccc(COC[C@H]2CCc3nc(c(-c4ccnc(N)n4)n23)-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C25H24FN5O2/c1-32-20-9-2-16(3-10-20)14-33-15-19-8-11-22-30-23(17-4-6-18(26)7-5-17)24(31(19)22)21-12-13-28-25(27)29-21/h2-7,9-10,12-13,19H,8,11,14-15H2,1H3,(H2,27,28,29)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against c-Jun N-terminal kinase 3 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50173168

(4-[(S)-2-(4-Fluoro-phenyl)-5-(4-methoxy-benzyloxym...)Show SMILES COc1ccc(COC[C@@H]2CCc3nc(c(-c4ccnc(N)n4)n23)-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C25H24FN5O2/c1-32-20-9-2-16(3-10-20)14-33-15-19-8-11-22-30-23(17-4-6-18(26)7-5-17)24(31(19)22)21-12-13-28-25(27)29-21/h2-7,9-10,12-13,19H,8,11,14-15H2,1H3,(H2,27,28,29)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38 at a concentration of 1.0 uM |
Bioorg Med Chem Lett 15: 4666-70 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.076
BindingDB Entry DOI: 10.7270/Q28G8K8K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data