

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2809 (Alkenyldiarylmethanes (ADAM) 6b | CHEMBL105859 | M...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2803 (Alkenyldiarylmethanes (ADAM) 50a | CHEMBL131761 | ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 499 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173819 (3-Chloro-5-[(E)-1-(3-cyano-phenyl)-5-methoxycarbon...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173813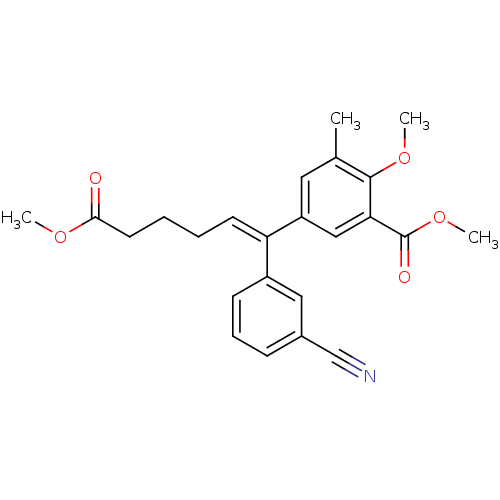 ((E)-5-[1-(3-cyanophenyl)-5-methoxycarbonylpent-1-e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173820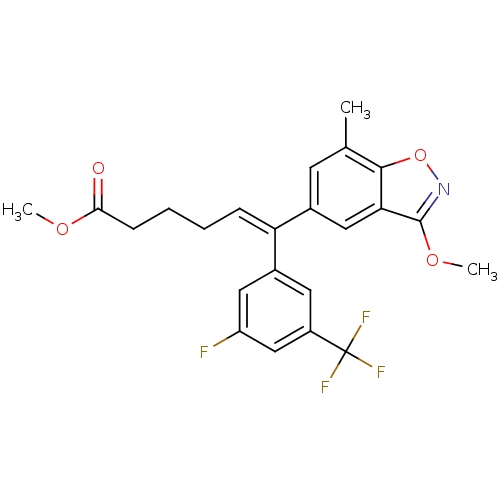 ((Z)-6-(3-Fluoro-5-trifluoromethyl-phenyl)-6-(3-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173810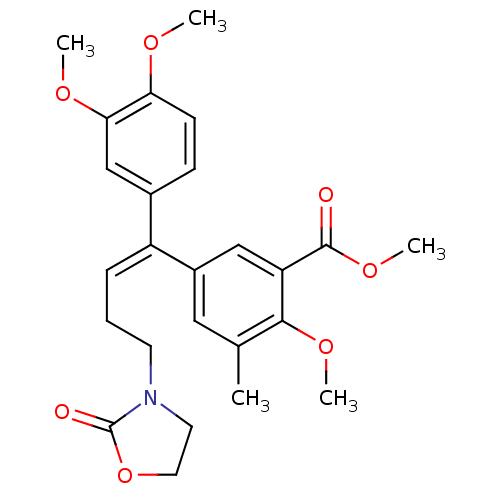 (5-[(E)-1-(3,4-Dimethoxy-phenyl)-4-(2-oxo-oxazolidi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173808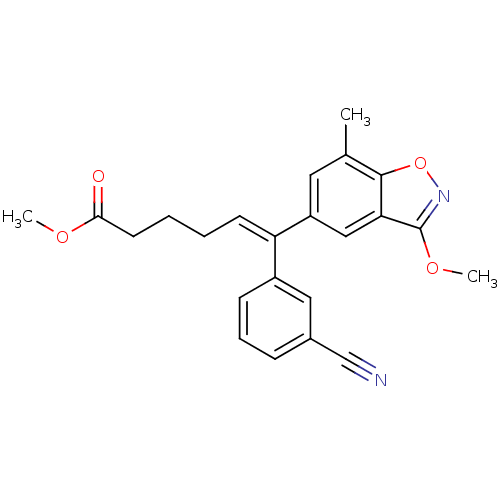 ((E)-6-(3-Cyano-phenyl)-6-(3-methoxy-7-methyl-benzo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173805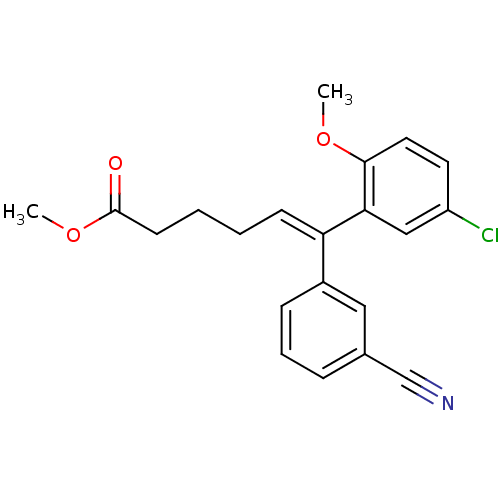 ((E)-6-(5-Chloro-2-methoxy-phenyl)-6-(3-cyano-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173811 ((Z)-6-(5-Chloro-2-methoxy-phenyl)-6-(2,7-dimethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173818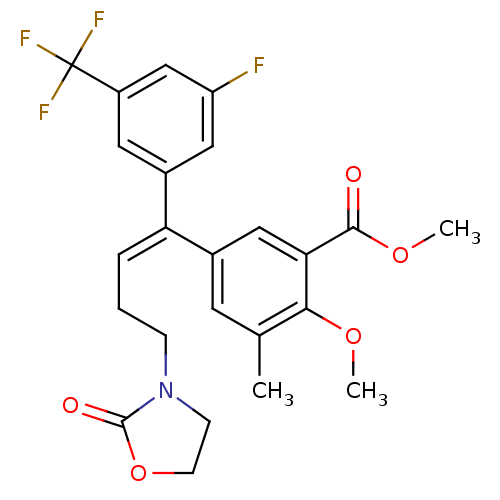 (5-[(E)-1-(3-Fluoro-5-trifluoromethyl-phenyl)-4-(2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173812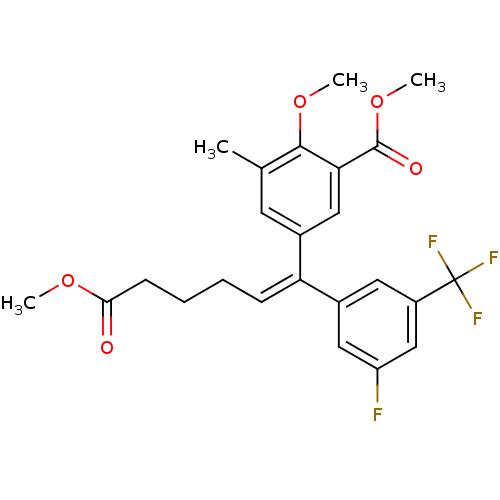 (5-[(E)-1-(3-Fluoro-5-trifluoromethyl-phenyl)-5-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173809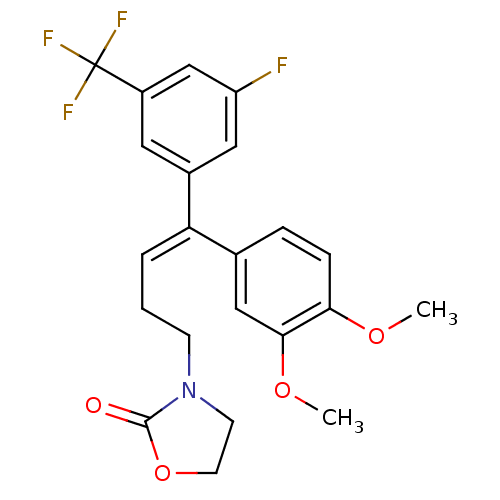 (3-[(E)-4-(3,4-Dimethoxy-phenyl)-4-(3-fluoro-5-trif...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173814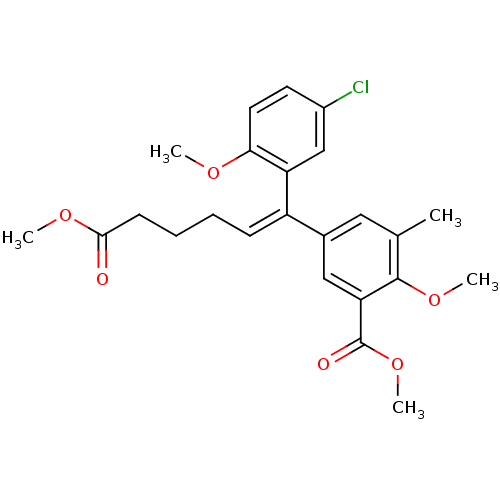 (5-[(Z)-1-(5-Chloro-2-methoxy-phenyl)-5-methoxycarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173816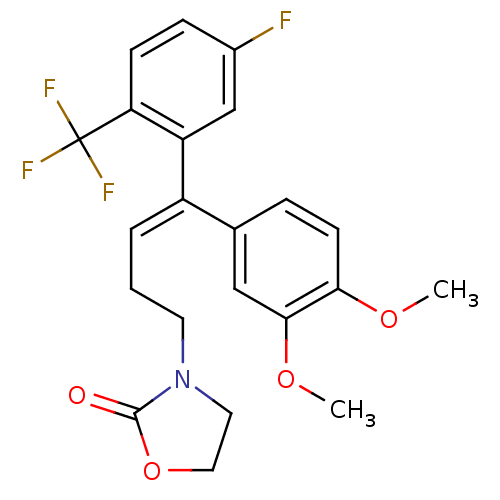 (3-[(E)-4-(3,4-Dimethoxy-phenyl)-4-(5-fluoro-2-trif...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173817 (3-Chloro-5-[(E)-1-(3-fluoro-5-trifluoromethyl-phen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173804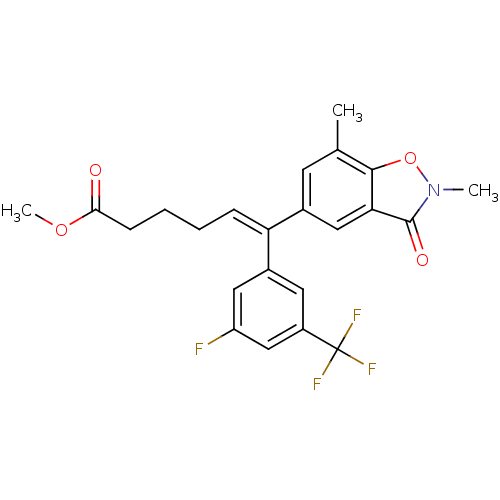 ((Z)-6-(2,7-Dimethyl-3-oxo-2,3-dihydro-benzo[d]isox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173807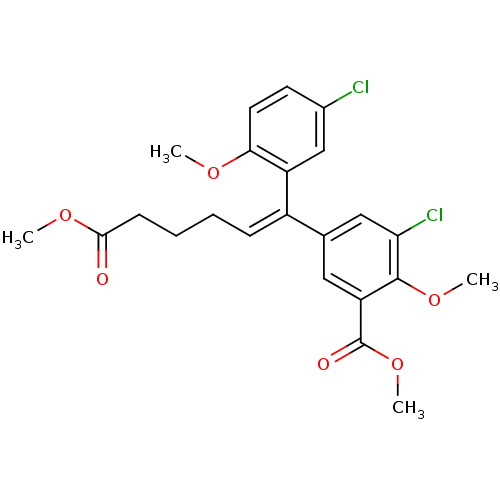 (3-Chloro-5-[(Z)-1-(5-chloro-2-methoxy-phenyl)-5-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173815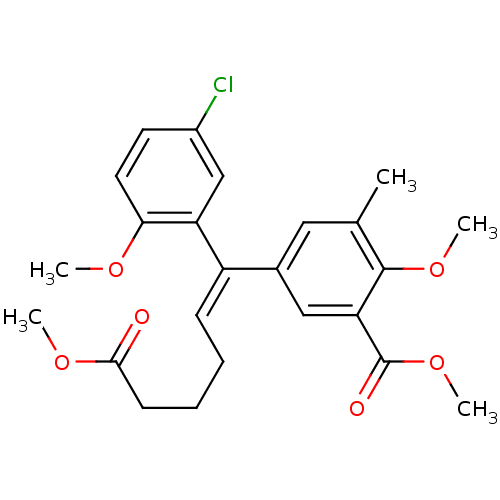 (5-[(E)-1-(5-Chloro-2-methoxy-phenyl)-5-methoxycarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50410542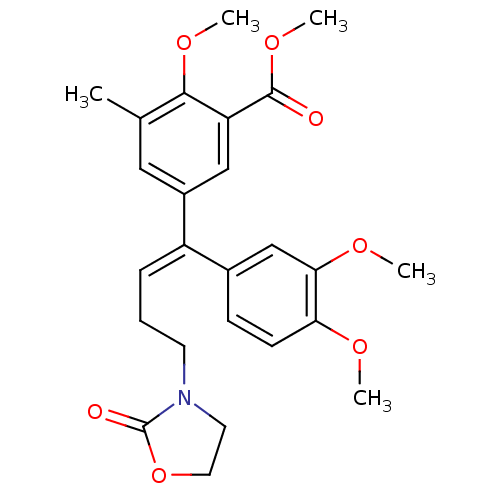 (CHEMBL2096827) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50173806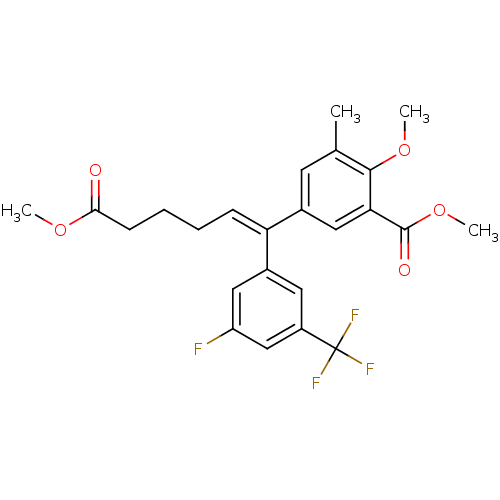 (5-[(Z)-1-(3-Fluoro-5-trifluoromethyl-phenyl)-5-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 reverse transcriptase activity | J Med Chem 48: 6140-55 (2005) Article DOI: 10.1021/jm050452s BindingDB Entry DOI: 10.7270/Q2TB16F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||