

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM9063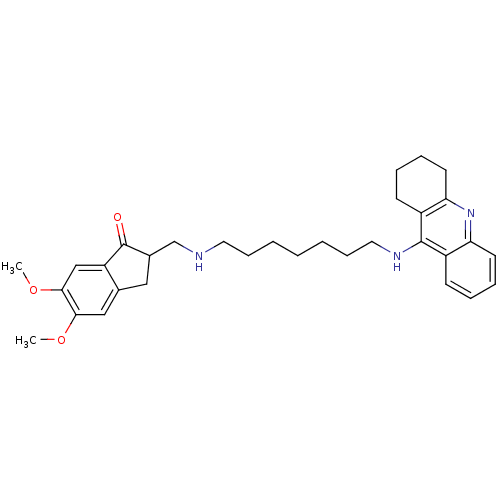 (5,6-Dimethoxy-2-{[7-(1,2,3,4-tetrahydro-acridin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9073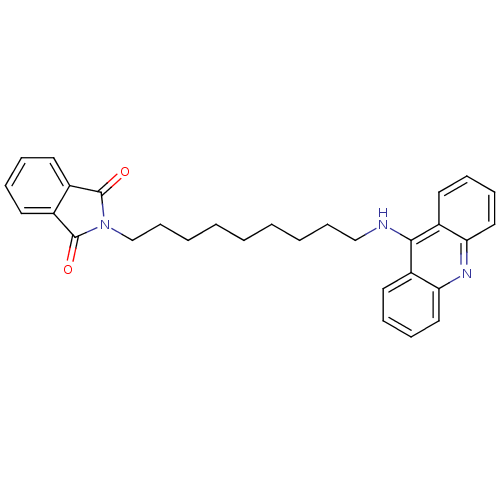 (2-[9-(Acridin-9-ylamino)-nonyl]-isoindole-1,3-dion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9067 (Donepezil-tacrine hybrid 12 | N-[4-({2-[(6-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9070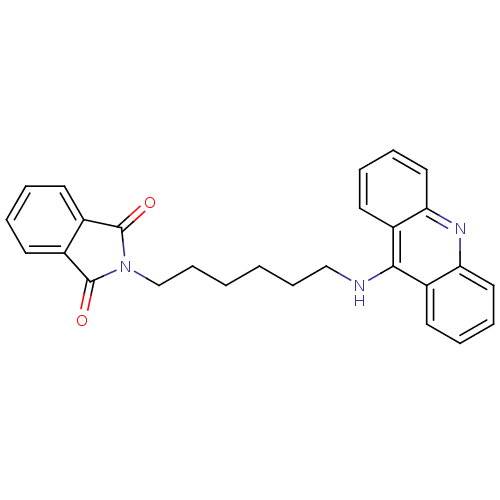 (2-[6-(Acridin-9-ylamino)-hexyl]-isoindole-1,3-dion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9068 (2-[7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9065 (Donepezil-tacrine hybrid 10 | N-[6-(6-Chloro-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9066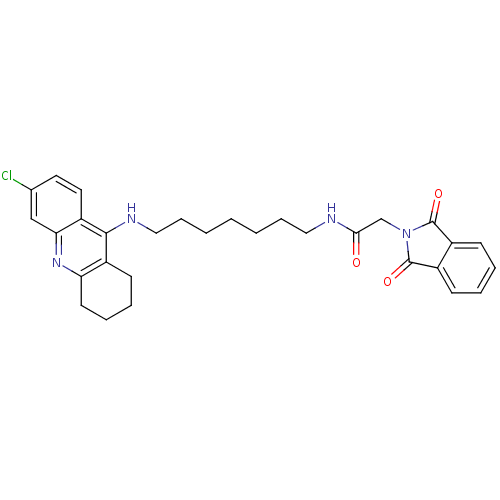 (Donepezil-tacrine hybrid 11 | N-[7-(6-Chloro-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9063 (5,6-Dimethoxy-2-{[7-(1,2,3,4-tetrahydro-acridin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9069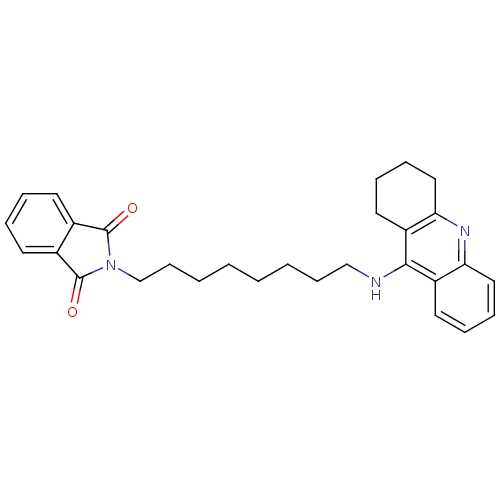 (2-[8-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-octyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9062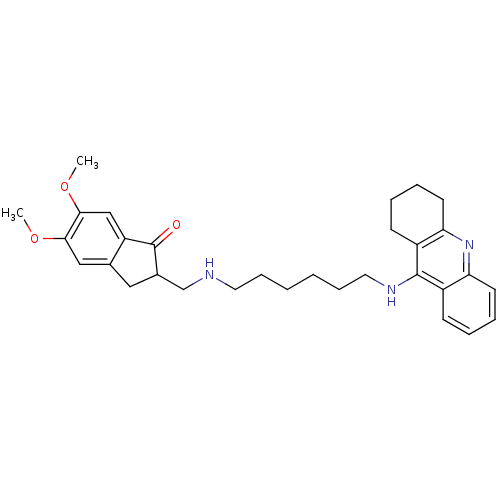 (5,6-Dimethoxy-2-{[6-(1,2,3,4-tetrahydro-acridin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9067 (Donepezil-tacrine hybrid 12 | N-[4-({2-[(6-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9073 (2-[9-(Acridin-9-ylamino)-nonyl]-isoindole-1,3-dion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9069 (2-[8-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-octyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9064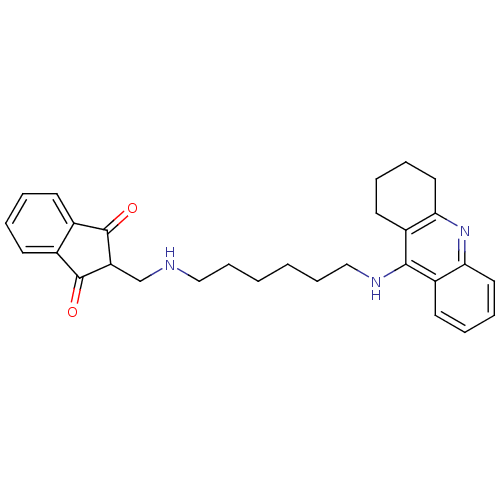 (2-({[6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9062 (5,6-Dimethoxy-2-{[6-(1,2,3,4-tetrahydro-acridin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9065 (Donepezil-tacrine hybrid 10 | N-[6-(6-Chloro-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9064 (2-({[6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 504 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9066 (Donepezil-tacrine hybrid 11 | N-[7-(6-Chloro-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9071 (2-[7-(Acridin-9-ylamino)-heptyl]-isoindole-1,3-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 711 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9070 (2-[6-(Acridin-9-ylamino)-hexyl]-isoindole-1,3-dion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9072 (2-[8-(Acridin-9-ylamino)-octyl]-isoindole-1,3-dion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9072 (2-[8-(Acridin-9-ylamino)-octyl]-isoindole-1,3-dion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9071 (2-[7-(Acridin-9-ylamino)-heptyl]-isoindole-1,3-dio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM9068 (2-[7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Neuropharma | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 405 nm were recorded for 10 min with... | Bioorg Med Chem 13: 6588-97 (2005) Article DOI: 10.1016/j.bmc.2005.09.029 BindingDB Entry DOI: 10.7270/Q2G73BW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||