

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11123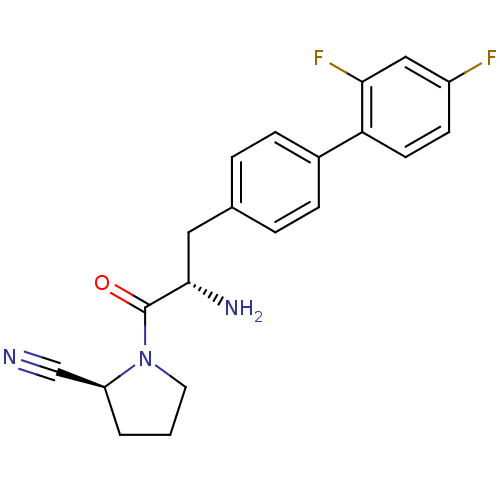 ((2S)-1-[(2S)-2-amino-3-[4-(2,4-difluorophenyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11121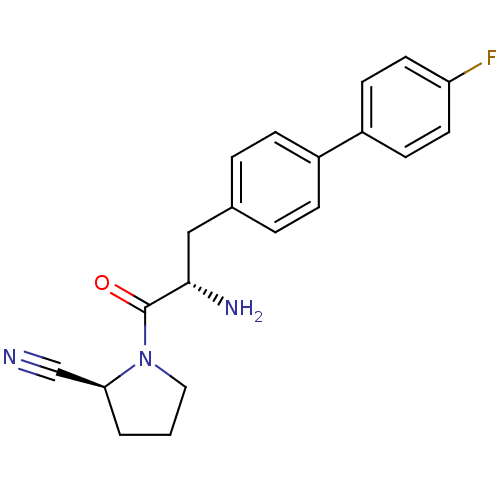 ((2S)-1-[(2S)-2-amino-3-[4-(4-fluorophenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11122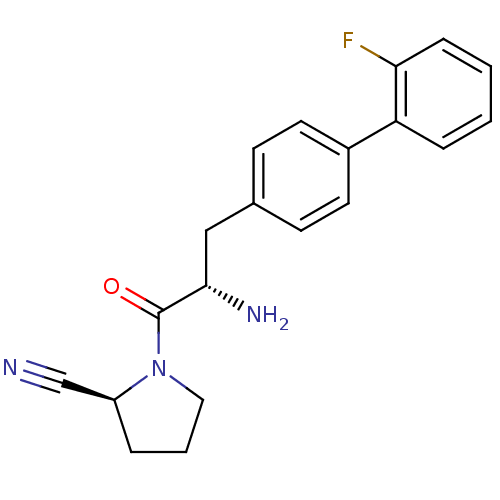 ((2S)-1-[(2S)-2-amino-3-[4-(2-fluorophenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11118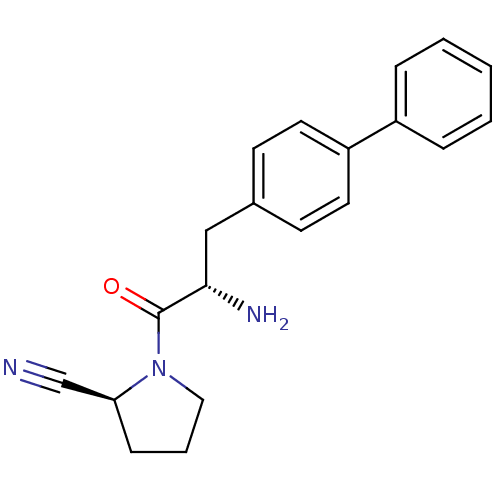 ((2S)-1-[(2S)-2-amino-3-(4-phenylphenyl)propanoyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11119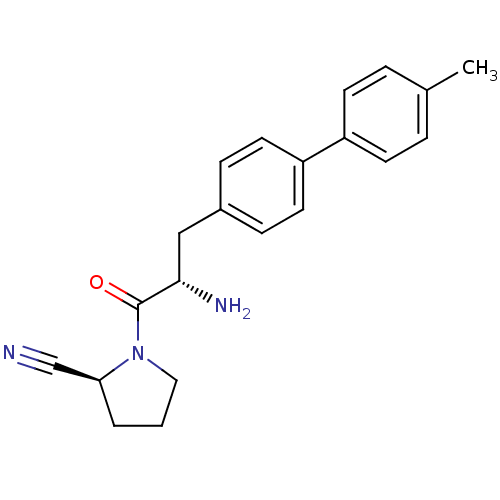 ((2S)-1-[(2S)-2-amino-3-[4-(4-methylphenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11120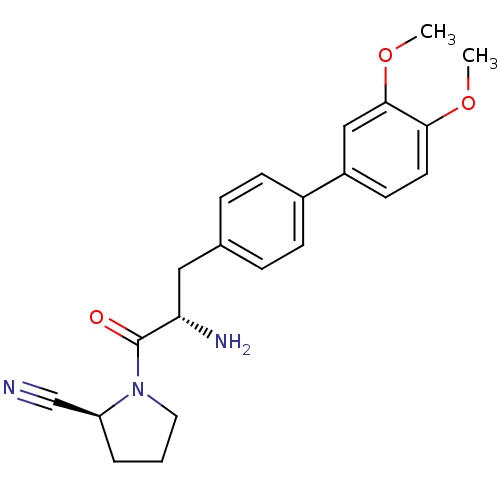 ((2S)-1-[(2S)-2-amino-3-[4-(3,4-dimethoxyphenyl)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11113 (6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11116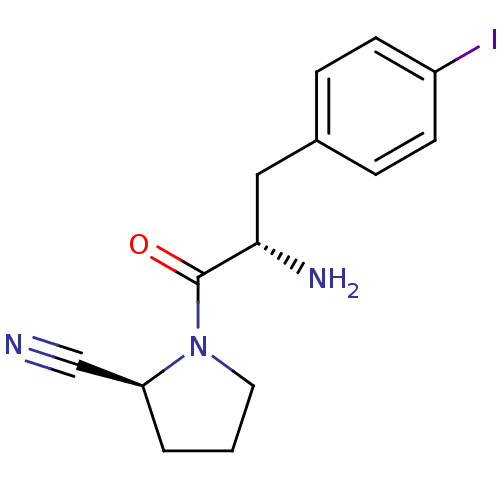 ((2S)-1-[(2S)-2-amino-3-(4-iodophenyl)propanoyl]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11124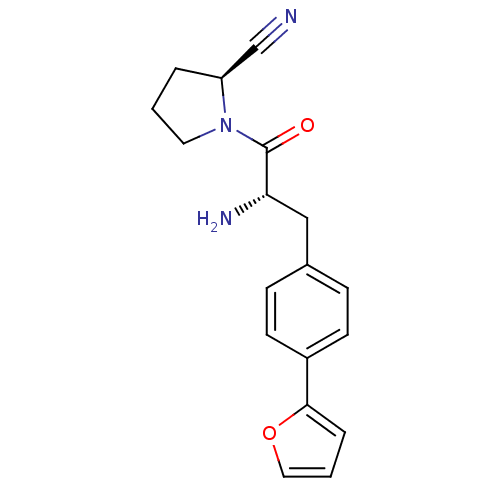 ((2S)-1-[(2S)-2-amino-3-[4-(furan-2-yl)phenyl]propa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 36 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11115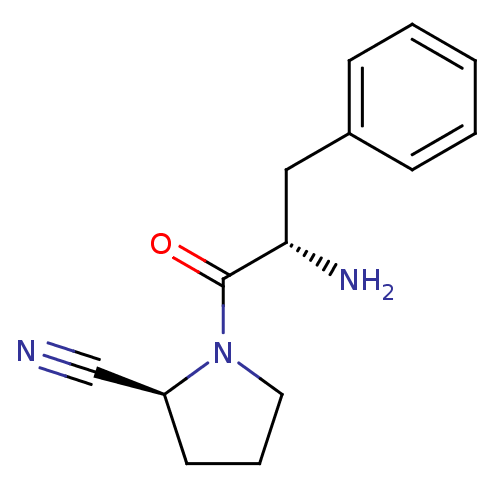 ((2S)-1-[(2S)-2-amino-3-phenylpropanoyl]pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 63 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11136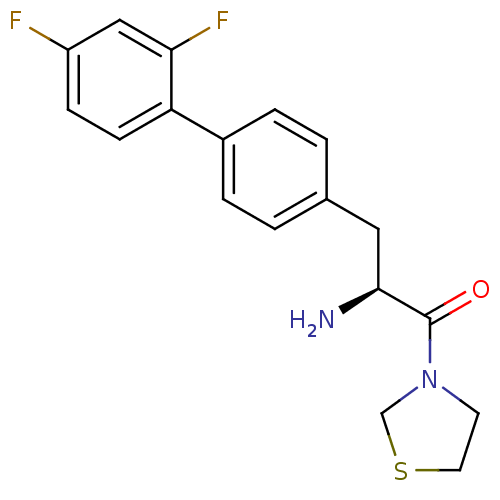 ((2S)-2-amino-3-[4-(2,4-difluorophenyl)phenyl]-1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 96 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11134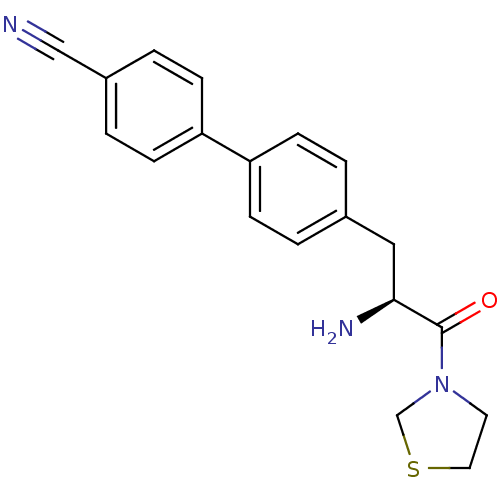 (4-{4-[(2S)-2-amino-3-oxo-3-(1,3-thiazolidin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11133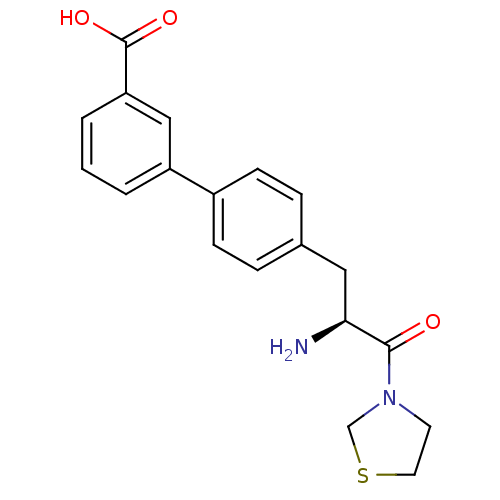 (3-{4-[(2S)-2-amino-3-oxo-3-(1,3-thiazolidin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 166 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11135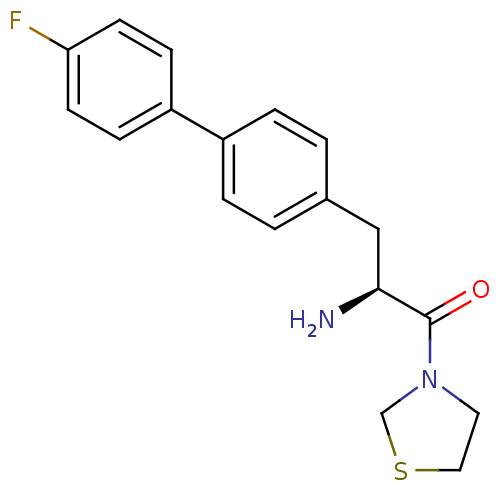 ((2S)-2-amino-3-[4-(4-fluorophenyl)phenyl]-1-(1,3-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 170 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11132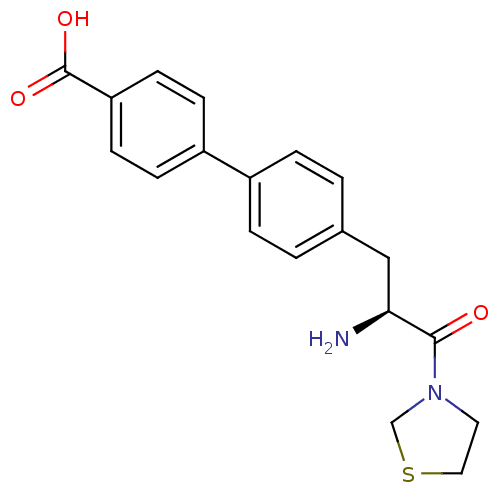 (4-{4-[(2S)-2-amino-3-oxo-3-(1,3-thiazolidin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11131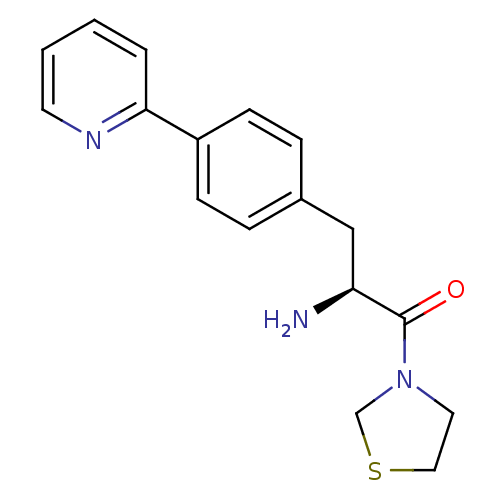 ((2S)-2-amino-3-[4-(pyridin-2-yl)phenyl]-1-(1,3-thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 355 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11125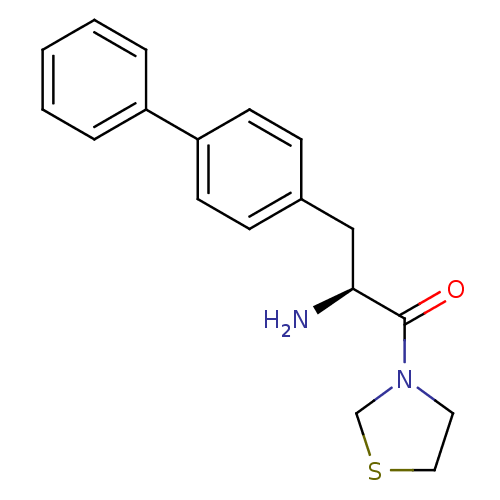 ((2S)-2-amino-3-(4-phenylphenyl)-1-(1,3-thiazolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 360 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11117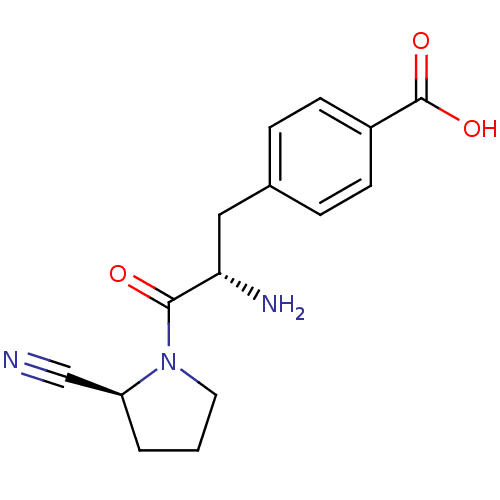 (4-[(2S)-2-amino-3-[(2S)-2-cyanopyrrolidin-1-yl]-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 470 | -36.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11130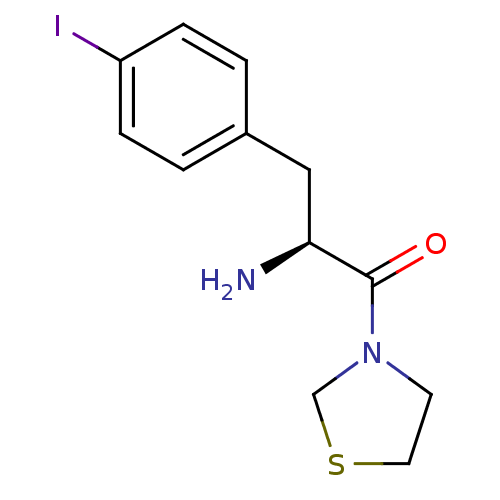 ((2S)-2-amino-3-(4-iodophenyl)-1-(1,3-thiazolidin-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 980 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11126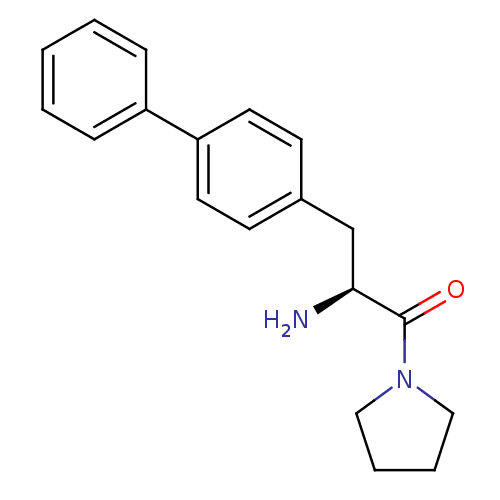 ((2S)-2-amino-3-(4-phenylphenyl)-1-(pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | -33.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11137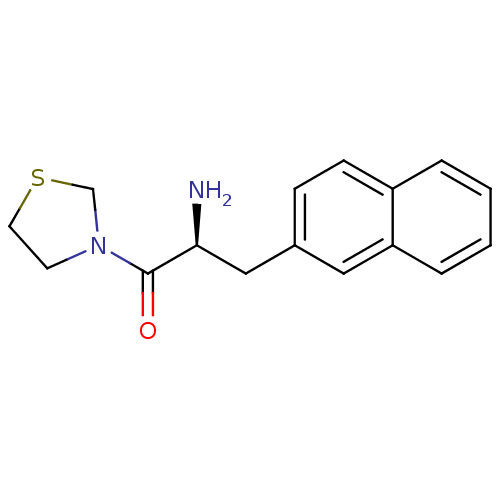 ((2S)-2-amino-3-(naphthalen-2-yl)-1-(1,3-thiazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.90E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11139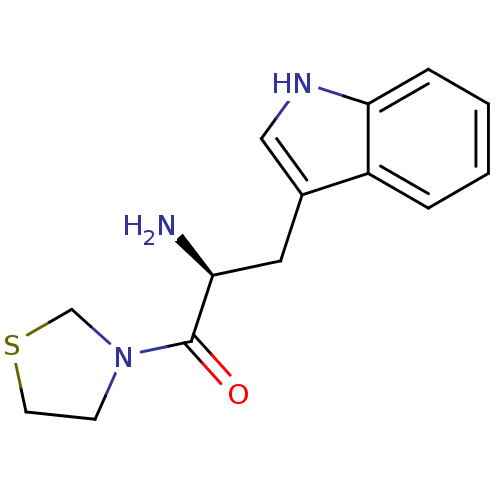 ((2S)-2-amino-3-(1H-indol-3-yl)-1-(1,3-thiazolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11129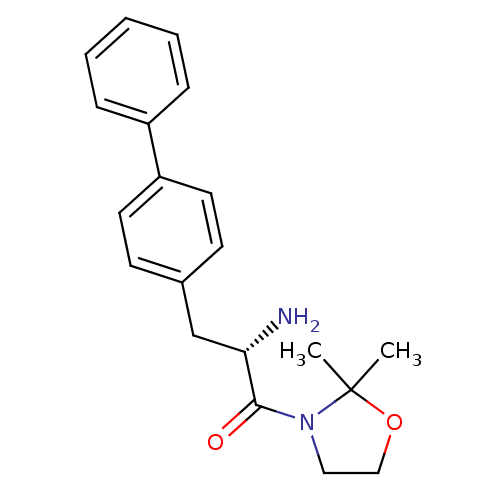 ((2S)-2-amino-1-(2,2-dimethyl-1,3-oxazolidin-3-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11138 ((2S)-2-amino-3-(naphthalen-1-yl)-1-(1,3-thiazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11127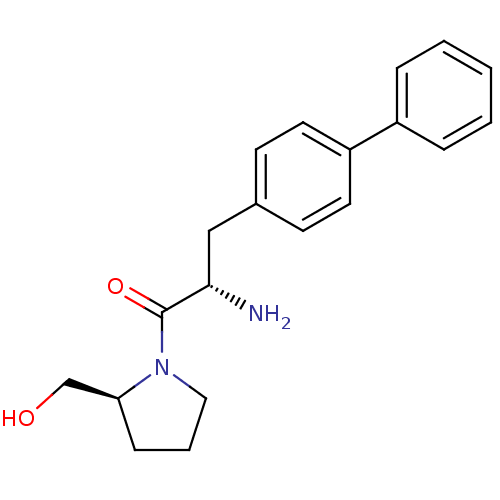 ((2S)-2-amino-1-[(2S)-2-(hydroxymethyl)pyrrolidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11128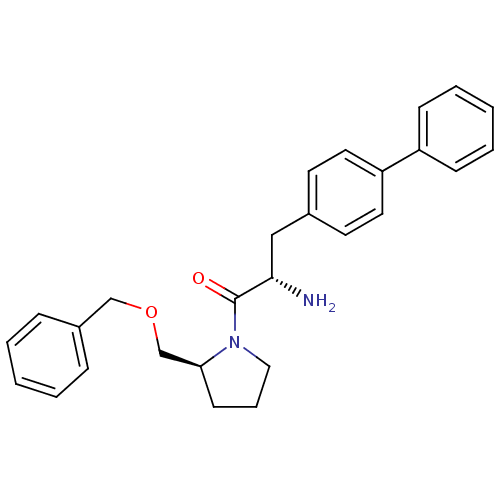 ((2S)-2-amino-1-[(2S)-2-[(benzyloxy)methyl]pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||