 Found 50 hits of Enzyme Inhibition Constant Data
Found 50 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50024321
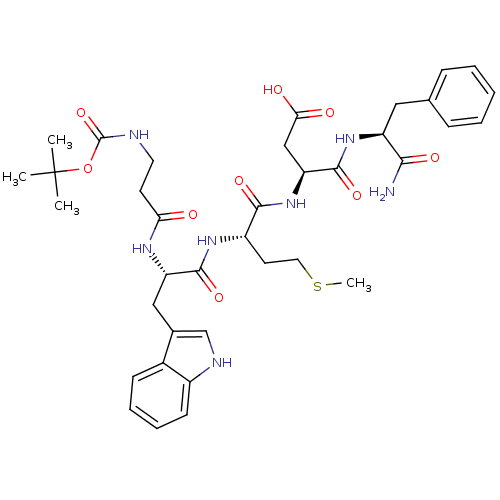
(3-{2-[2-(3-tert-Butoxycarbonylamino-propionylamino...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H49N7O9S/c1-37(2,3)53-36(52)39-16-14-30(45)41-28(19-23-21-40-25-13-9-8-12-24(23)25)34(50)42-26(15-17-54-4)33(49)44-29(20-31(46)47)35(51)43-27(32(38)48)18-22-10-6-5-7-11-22/h5-13,21,26-29,40H,14-20H2,1-4H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50455595

(CHEMBL2112338)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCS([O-])(=O)=O)c1ccccc1 |TLB:18:19:23:16.17.22,15:16:23:19.25.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,18:17:23:19.25.20| Show InChI InChI=1S/C34H42N4O7S.Na/c1-34(18-26-19-35-28-10-6-5-9-27(26)28,38-33(41)45-31-24-14-21-13-22(16-24)17-25(31)15-21)32(40)36-20-29(23-7-3-2-4-8-23)37-30(39)11-12-46(42,43)44;/h2-10,19,21-22,24-25,29,31,35H,11-18,20H2,1H3,(H,36,40)(H,37,39)(H,38,41)(H,42,43,44);/q;+1/p-1/t21?,22?,24?,25?,29-,31?,34+;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50455596

(CHEMBL2112336)Show SMILES [H][C@](CNC(=O)[C@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)C[S+]([O-])c1nc[nH]n1)c1ccccc1 |wU:1.36,wD:6.7,1.0,TLB:21:22:24:27.28.26,THB:26:25:22:27.29.28,26:27:22:25.24.31,29:30:24:27.28.26,29:27:24:22.30.31,(5.59,-7.16,;4.27,-7.93,;2.94,-8.7,;1.6,-7.94,;.28,-8.71,;.28,-10.24,;-1.05,-7.94,;-1.06,-9.47,;-1.04,-6.39,;-1.93,-5.12,;-3.45,-5.35,;-4.13,-3.97,;-3.05,-2.89,;-3.1,-1.35,;-1.81,-.52,;-.44,-1.24,;-.37,-2.78,;-1.67,-3.6,;-2.39,-8.69,;-3.72,-7.93,;-3.71,-6.39,;-5.07,-8.67,;-6.39,-7.9,;-6.39,-6.36,;-7.71,-5.57,;-9.05,-6.32,;-11.02,-6.39,;-9.68,-7.16,;-7.82,-7.41,;-9.28,-8.64,;-7.74,-8.65,;-9.07,-7.87,;4.26,-6.39,;5.6,-5.61,;5.58,-4.07,;6.93,-6.37,;8.27,-5.59,;8.27,-4.05,;9.61,-6.36,;9.79,-7.88,;11.3,-8.21,;12.07,-6.85,;11.03,-5.72,;5.6,-8.69,;5.61,-10.24,;6.94,-11.01,;8.28,-10.22,;8.26,-8.67,;6.93,-7.92,)| Show InChI InChI=1S/C35H41N7O5S/c1-35(16-26-17-36-28-10-6-5-9-27(26)28,41-34(45)47-31-24-12-21-11-22(14-24)15-25(31)13-21)32(44)37-18-29(23-7-3-2-4-8-23)40-30(43)19-48(46)33-38-20-39-42-33/h2-10,17,20-22,24-25,29,31,36H,11-16,18-19H2,1H3,(H,37,44)(H,40,43)(H,41,45)(H,38,39,42)/t21?,22?,24?,25?,29-,31?,35+,48?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-B receptor by displacing [125I]bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006875

(CHEMBL263969 | N-{(S)-2-[(R)-2-(Adamantan-2-yloxyc...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCC(O)=O)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,(5.02,-9.99,;5.02,-8.45,;5.04,-6.91,;4.14,-5.65,;2.62,-5.88,;1.94,-4.5,;3.02,-3.41,;2.96,-1.87,;4.25,-1.05,;5.63,-1.76,;5.69,-3.3,;4.39,-4.13,;3.69,-9.22,;2.36,-8.45,;2.36,-6.91,;1.01,-9.22,;-.32,-8.43,;-.32,-6.89,;-1.63,-6.11,;-2.98,-6.87,;-4.94,-6.94,;-3.61,-7.71,;-2.12,-7.3,;-3.2,-9.2,;-1.66,-9.2,;-2.99,-8.41,;6.35,-9.22,;6.35,-10.76,;7.68,-8.45,;9.03,-9.22,;10.34,-8.43,;10.34,-6.89,;11.67,-6.11,;11.65,-4.57,;13,-6.87,;14.33,-6.07,;15.66,-6.84,;16.99,-6.05,;15.69,-8.38,;11.69,-9.2,;11.7,-10.74,;13.03,-11.5,;14.36,-10.72,;14.35,-9.18,;13,-8.41,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t21?,22?,24?,25?,29-,32?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50455598

(CHEMBL2112335)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCn1nn[nH]c1=S)c1ccccc1 |TLB:25:24:22:19.18.20,THB:15:16:22:19.18.20,20:19:16:21.23.22,20:21:16:19.18.25,25:19:16.24.23:22| Show InChI InChI=1S/C35H42N8O4S/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(46)47-31-24-14-21-13-22(16-24)17-25(31)15-21)32(45)37-20-29(23-7-3-2-4-8-23)38-30(44)11-12-43-33(48)40-41-42-43/h2-10,19,21-22,24-25,29,31,36H,11-18,20H2,1H3,(H,37,45)(H,38,44)(H,39,46)(H,40,42,48)/t21?,22?,24?,25?,29-,31?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-B receptor by displacing [125I]bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50455600

(CHEMBL2112330)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CSc1nc[nH]n1)c1ccccc1 |TLB:15:16:23:19.25.20,18:19:16.17.22:23,THB:20:19:16:21.22.23,20:21:16:19.18.25,18:17:23:19.25.20| Show InChI InChI=1S/C35H41N7O4S/c1-35(16-26-17-36-28-10-6-5-9-27(26)28,41-34(45)46-31-24-12-21-11-22(14-24)15-25(31)13-21)32(44)37-18-29(23-7-3-2-4-8-23)40-30(43)19-47-33-38-20-39-42-33/h2-10,17,20-22,24-25,29,31,36H,11-16,18-19H2,1H3,(H,37,44)(H,40,43)(H,41,45)(H,38,39,42)/t21?,22?,24?,25?,29-,31?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50455597

(CHEMBL2112334)Show SMILES [H][C@](CNC(=O)[C@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)Cc1cc(O)no1)c1ccccc1 |wU:1.36,wD:6.7,1.0,TLB:21:22:29:25.31.26,24:25:22.23.28:29,THB:26:25:22:27.28.29,26:27:22:25.24.31,24:23:29:25.31.26,(18.39,-8.83,;17.06,-9.61,;15.75,-10.38,;14.41,-9.63,;13.08,-10.4,;13.08,-11.93,;11.76,-9.63,;11.74,-11.17,;11.76,-8.09,;10.87,-6.81,;9.34,-7.05,;8.66,-5.68,;9.75,-4.6,;9.68,-3.05,;10.97,-2.22,;12.35,-2.94,;12.41,-4.48,;11.11,-5.3,;10.42,-10.38,;9.09,-9.63,;9.09,-8.09,;7.74,-10.37,;6.42,-9.61,;5.07,-10.36,;3.54,-10.36,;3.12,-8.88,;1.79,-8.11,;3.75,-8.04,;3.75,-9.59,;5.09,-7.28,;6.42,-8.06,;4.61,-8.74,;17.05,-8.06,;18.38,-7.28,;18.37,-5.75,;19.7,-8.04,;21.05,-7.26,;21.2,-5.71,;22.7,-5.39,;23.33,-3.97,;23.5,-6.71,;22.47,-7.85,;18.39,-10.36,;19.73,-9.59,;21.06,-10.34,;21.08,-11.88,;19.75,-12.67,;18.41,-11.9,)| Show InChI InChI=1S/C36H41N5O6/c1-36(18-26-19-37-29-10-6-5-9-28(26)29,40-35(45)46-33-24-12-21-11-22(14-24)15-25(33)13-21)34(44)38-20-30(23-7-3-2-4-8-23)39-31(42)16-27-17-32(43)41-47-27/h2-10,17,19,21-22,24-25,30,33,37H,11-16,18,20H2,1H3,(H,38,44)(H,39,42)(H,40,45)(H,41,43)/t21?,22?,24?,25?,30-,33?,36+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-B receptor by displacing [125I]bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50455599

(CHEMBL2112333)Show SMILES [H][C@](CNC(=O)[C@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CSc1n[nH]c(=O)[nH]1)c1ccccc1 |wU:1.36,wD:6.7,1.0,TLB:21:22:24:27.28.26,THB:26:25:22:27.29.28,26:27:22:25.24.31,29:30:24:27.28.26,29:27:24:22.30.31,(14.15,-7.57,;12.83,-8.34,;11.5,-9.12,;10.17,-8.37,;8.85,-9.14,;8.85,-10.68,;7.52,-8.37,;7.52,-9.91,;7.52,-6.84,;6.63,-5.56,;5.11,-5.79,;4.43,-4.41,;5.51,-3.33,;5.46,-1.8,;6.74,-.97,;8.11,-1.69,;8.18,-3.22,;6.88,-4.04,;6.18,-9.13,;4.85,-8.37,;4.85,-6.82,;3.51,-9.12,;2.19,-8.34,;2.19,-6.8,;.87,-6.03,;-.47,-6.79,;-2.44,-6.85,;-1.1,-7.62,;.87,-7.93,;-.69,-9.11,;.84,-9.11,;-.48,-8.32,;12.82,-6.81,;14.15,-6.03,;14.13,-4.48,;15.49,-6.77,;16.82,-6,;18.16,-6.77,;18.34,-8.29,;19.86,-8.6,;20.61,-7.26,;22.15,-7.08,;19.58,-6.12,;14.17,-9.11,;14.17,-10.64,;15.51,-11.41,;16.84,-10.63,;16.82,-9.09,;15.49,-8.32,)| Show InChI InChI=1S/C35H41N7O5S/c1-35(16-25-17-36-27-10-6-5-9-26(25)27,40-34(46)47-30-23-12-20-11-21(14-23)15-24(30)13-20)31(44)37-18-28(22-7-3-2-4-8-22)38-29(43)19-48-33-39-32(45)41-42-33/h2-10,17,20-21,23-24,28,30,36H,11-16,18-19H2,1H3,(H,37,44)(H,38,43)(H,40,46)(H2,39,41,42,45)/t20?,21?,23?,24?,28-,30?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006881
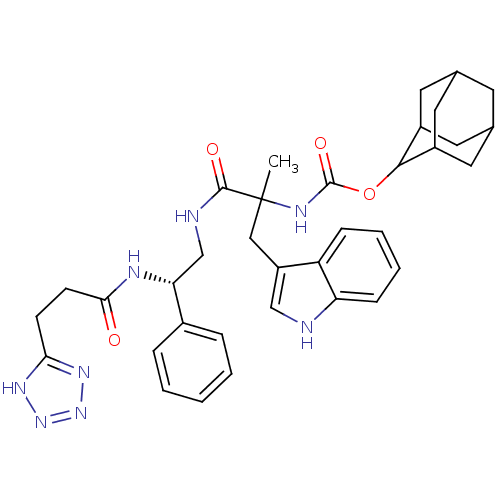
(CHEMBL88090 | {2-(1H-Indol-3-yl)-1-methyl-1-[2-phe...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCc1nnn[nH]1)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25,(10.08,-15.84,;10.09,-14.29,;10.1,-12.76,;9.2,-11.48,;7.68,-11.72,;7,-10.34,;8.09,-9.26,;8.02,-7.73,;9.31,-6.89,;10.69,-7.61,;10.75,-9.14,;9.45,-9.96,;8.76,-15.06,;7.42,-14.29,;7.42,-12.75,;6.07,-15.04,;4.75,-14.27,;4.75,-12.73,;3.43,-11.95,;2.09,-12.72,;.13,-12.77,;1.46,-13.55,;2.95,-13.14,;1.87,-15.04,;3.4,-15.04,;2.08,-14.25,;11.41,-15.07,;11.41,-16.61,;12.75,-14.29,;14.08,-15.04,;15.4,-14.27,;15.39,-12.74,;16.72,-11.95,;16.71,-10.41,;18.06,-12.7,;19.39,-11.93,;20.74,-12.69,;22.28,-12.65,;22.8,-14.11,;21.58,-15.04,;20.29,-14.17,;16.73,-15.04,;16.75,-16.57,;18.09,-17.34,;19.42,-16.56,;19.4,-15.01,;18.07,-14.26,)| Show InChI InChI=1S/C35H42N8O4/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(46)47-32-24-14-21-13-22(16-24)17-25(32)15-21)33(45)37-20-29(23-7-3-2-4-8-23)38-31(44)12-11-30-40-42-43-41-30/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,45)(H,38,44)(H,39,46)(H,40,41,42,43)/t21?,22?,24?,25?,29-,32?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006885
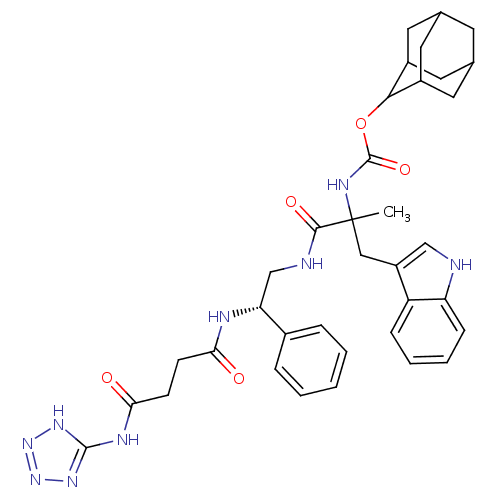
((2-(1H-Indol-3-yl)-1-methyl-1-{2-phenyl-2-[3-(5H-t...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCC(=O)Nc1nnn[nH]1)c1ccccc1 |wU:30.35,TLB:25:24:22:19.18.20,THB:15:16:22:19.18.20,20:19:16:21.22.23,20:21:16:19.18.25,25:19:22:16.24.23,(5.37,-5.89,;5.39,-4.34,;5.4,-2.81,;4.5,-1.53,;2.98,-1.76,;2.3,-.38,;3.38,.69,;3.31,2.23,;4.6,3.06,;5.98,2.34,;6.04,.81,;4.74,-.01,;4.05,-5.11,;2.72,-4.33,;2.72,-2.8,;1.37,-5.09,;.05,-4.32,;.05,-2.77,;-1.76,-3.17,;-3.25,-3.58,;-4.58,-2.82,;-2.62,-2.76,;-1.28,-2,;-2.62,-4.29,;-1.3,-5.08,;-2.83,-5.08,;6.71,-5.11,;6.71,-6.65,;8.04,-4.33,;9.38,-5.09,;10.69,-4.32,;10.68,-2.79,;12.02,-2,;12,-.45,;13.35,-2.75,;14.69,-1.98,;16.03,-2.74,;16.05,-4.29,;17.37,-1.95,;18.7,-2.72,;20.12,-2.07,;21.16,-3.21,;20.4,-4.55,;18.89,-4.25,;12.02,-5.08,;12.04,-6.61,;13.38,-7.39,;14.71,-6.61,;14.7,-5.06,;13.36,-4.31,)| Show InChI InChI=1S/C36H43N9O5/c1-36(18-26-19-37-28-10-6-5-9-27(26)28,41-35(49)50-32-24-14-21-13-22(16-24)17-25(32)15-21)33(48)38-20-29(23-7-3-2-4-8-23)39-30(46)11-12-31(47)40-34-42-44-45-43-34/h2-10,19,21-22,24-25,29,32,37H,11-18,20H2,1H3,(H,38,48)(H,39,46)(H,41,49)(H2,40,42,43,44,45,47)/t21?,22?,24?,25?,29-,32?,36?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006889
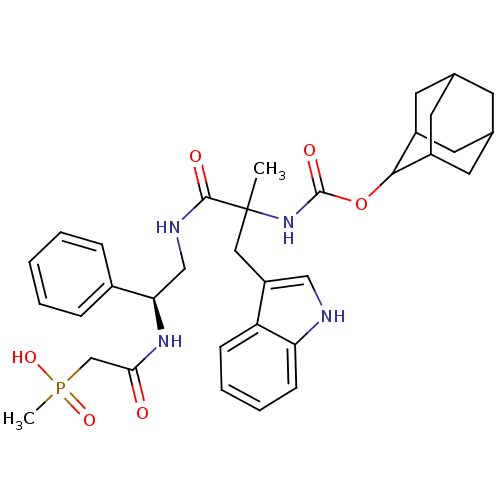
(({2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-indo...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CP(C)(O)=O)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,(11.35,-13.19,;11.36,-11.65,;11.37,-10.11,;10.48,-8.83,;8.94,-9.06,;8.27,-7.68,;9.36,-6.61,;9.29,-5.07,;10.58,-4.23,;11.95,-4.95,;12.02,-6.49,;10.72,-7.31,;10.02,-12.41,;8.69,-11.64,;8.69,-10.1,;7.35,-12.4,;6.02,-11.63,;6.02,-10.08,;4.7,-9.29,;3.36,-10.06,;1.38,-10.13,;2.73,-10.9,;4.21,-10.48,;3.13,-12.39,;4.67,-12.39,;3.34,-11.6,;12.69,-12.42,;12.69,-13.96,;14.02,-11.64,;15.36,-12.4,;16.68,-11.63,;16.67,-10.09,;18,-9.29,;17.97,-7.75,;19.33,-10.06,;20.66,-9.27,;21.59,-10.51,;21.87,-8.33,;19.72,-8.03,;18.02,-12.39,;18.02,-13.93,;19.37,-14.68,;20.7,-13.91,;20.68,-12.37,;19.34,-11.6,)| Show InChI InChI=1S/C34H43N4O6P/c1-34(17-26-18-35-28-11-7-6-10-27(26)28,38-33(41)44-31-24-13-21-12-22(15-24)16-25(31)14-21)32(40)36-19-29(23-8-4-3-5-9-23)37-30(39)20-45(2,42)43/h3-11,18,21-22,24-25,29,31,35H,12-17,19-20H2,1-2H3,(H,36,40)(H,37,39)(H,38,41)(H,42,43)/t21?,22?,24?,25?,29-,31?,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006877

(({2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-indo...)Show SMILES CCOP(O)(=O)CC(=O)N[C@H](CNC(=O)C(C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)c1ccccc1 |wU:10.9,TLB:30:31:33:36.37.35,THB:38:39:33:36.37.35,38:36:33:31.39.40,35:34:31:36.38.37,35:36:31:34.33.40,(26.18,-4.98,;24.95,-4.04,;23.53,-4.63,;22.31,-3.69,;23.53,-2.75,;21.37,-2.46,;20.98,-4.48,;19.65,-3.72,;19.63,-2.18,;18.32,-4.51,;18.34,-6.05,;17.02,-6.82,;15.68,-6.07,;14.35,-6.84,;14.35,-8.38,;13.02,-6.07,;13,-7.61,;13.03,-4.53,;12.13,-3.25,;10.6,-3.48,;9.92,-2.11,;11.01,-1.03,;10.95,.51,;12.23,1.35,;13.61,.62,;13.68,-.92,;12.37,-1.73,;11.67,-6.84,;10.34,-6.07,;10.34,-4.53,;9.01,-6.82,;7.68,-6.05,;7.68,-4.5,;6.35,-3.72,;5.02,-4.49,;3.04,-4.55,;4.39,-5.32,;5.86,-4.91,;4.79,-6.81,;6.33,-6.81,;5,-6.03,;19.68,-6.81,;21,-6.03,;22.34,-6.79,;22.35,-8.34,;21.02,-9.11,;19.68,-8.35,)| Show InChI InChI=1S/C35H45N4O7P/c1-3-45-47(43,44)21-31(40)38-30(24-9-5-4-6-10-24)20-37-33(41)35(2,18-27-19-36-29-12-8-7-11-28(27)29)39-34(42)46-32-25-14-22-13-23(16-25)17-26(32)15-22/h4-12,19,22-23,25-26,30,32,36H,3,13-18,20-21H2,1-2H3,(H,37,41)(H,38,40)(H,39,42)(H,43,44)/t22?,23?,25?,26?,30-,32?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006883

(CHEMBL313499 | [1-[2-(3-Hydroxycarbamoyl-propionyl...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCC(=O)NO)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,(9.29,-10.11,;9.29,-8.57,;9.29,-7.02,;8.4,-5.75,;6.88,-5.98,;6.2,-4.6,;7.28,-3.53,;7.22,-1.98,;8.51,-1.15,;9.88,-1.86,;9.95,-3.41,;8.65,-4.23,;7.95,-9.32,;6.62,-8.56,;6.62,-7.02,;5.28,-9.31,;3.95,-8.54,;3.95,-7,;2.63,-6.21,;1.29,-6.97,;-.68,-7.04,;.66,-7.81,;2.13,-7.4,;1.06,-9.29,;2.6,-9.29,;1.27,-8.52,;10.62,-9.33,;10.62,-10.87,;11.94,-8.56,;13.28,-9.32,;14.61,-8.54,;14.6,-7,;15.93,-6.21,;15.91,-4.67,;17.27,-6.97,;18.61,-6.19,;19.94,-6.95,;19.97,-8.5,;21.28,-6.17,;22.63,-6.93,;15.95,-9.29,;15.96,-10.84,;17.29,-11.61,;18.63,-10.82,;18.61,-9.27,;17.27,-8.52,)| Show InChI InChI=1S/C35H43N5O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)46-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(41)11-12-31(42)40-45/h2-10,19,21-22,24-25,29,32,36,45H,11-18,20H2,1H3,(H,37,43)(H,38,41)(H,39,44)(H,40,42)/t21?,22?,24?,25?,29-,32?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006868
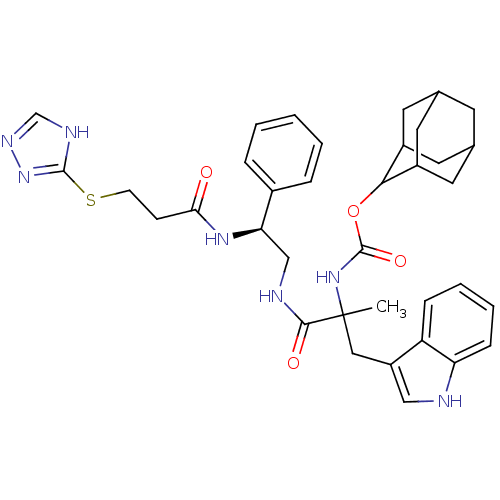
((2-(1H-Indol-3-yl)-1-methyl-1-{2-phenyl-2-[3-(2H-[...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCSc1nnc[nH]1)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25,(12.99,-8.61,;12.99,-7.06,;12.99,-5.53,;12.1,-4.25,;10.58,-4.49,;9.9,-3.1,;10.98,-2.02,;10.92,-.49,;12.21,.34,;13.58,-.38,;13.65,-1.91,;12.35,-2.73,;11.65,-7.83,;10.32,-7.06,;10.32,-5.52,;8.98,-7.81,;7.65,-7.04,;7.65,-5.49,;6.33,-4.72,;4.99,-5.49,;3.02,-5.54,;4.34,-6.31,;5.84,-5.91,;4.77,-7.81,;6.31,-7.81,;4.98,-7.02,;14.32,-7.84,;14.32,-9.38,;15.64,-7.06,;16.98,-7.81,;18.31,-7.04,;18.29,-5.51,;19.63,-4.72,;19.6,-3.17,;20.96,-5.47,;22.3,-4.7,;23.65,-5.45,;25,-6.22,;25.18,-7.74,;26.68,-8.05,;27.45,-6.71,;26.4,-5.56,;19.64,-7.81,;19.65,-9.34,;20.99,-10.11,;22.32,-9.33,;22.3,-7.78,;20.96,-7.03,)| Show InChI InChI=1S/C36H43N7O4S/c1-36(18-27-19-37-29-10-6-5-9-28(27)29,42-35(46)47-32-25-14-22-13-23(16-25)17-26(32)15-22)33(45)38-20-30(24-7-3-2-4-8-24)41-31(44)11-12-48-34-39-21-40-43-34/h2-10,19,21-23,25-26,30,32,37H,11-18,20H2,1H3,(H,38,45)(H,41,44)(H,42,46)(H,39,40,43)/t22?,23?,25?,26?,30-,32?,36?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50455601

(CHEMBL2112331)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CSc1c[nH]nn1)c1ccccc1 |TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25| Show InChI InChI=1S/C35H41N7O4S/c1-35(16-26-17-36-28-10-6-5-9-27(26)28,40-34(45)46-32-24-12-21-11-22(14-24)15-25(32)13-21)33(44)37-18-29(23-7-3-2-4-8-23)39-30(43)20-47-31-19-38-42-41-31/h2-10,17,19,21-22,24-25,29,32,36H,11-16,18,20H2,1H3,(H,37,44)(H,39,43)(H,40,45)(H,38,41,42)/t21?,22?,24?,25?,29-,32?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-B receptor by displacing [125I]bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006882

(CHEMBL312894 | {2-(1H-Indol-3-yl)-1-[2-(3-methoxyc...)Show SMILES CONC(=O)CCC(=O)N[C@H](CNC(=O)C(C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)c1ccccc1 |wU:10.9,TLB:30:31:33:36.37.35,THB:38:39:33:36.37.35,38:36:33:31.39.40,35:34:31:36.38.37,35:36:31:34.33.40,(24.33,-6.87,;24.3,-5.33,;22.97,-4.55,;21.63,-5.35,;21.65,-6.9,;20.29,-4.58,;18.95,-5.36,;17.62,-4.6,;17.6,-3.07,;16.28,-5.4,;16.29,-6.93,;14.98,-7.71,;13.64,-6.94,;12.31,-7.72,;12.31,-9.26,;10.99,-6.96,;10.97,-8.49,;11,-5.42,;10.1,-4.13,;8.57,-4.37,;7.9,-3,;8.98,-1.92,;8.91,-.38,;10.2,.46,;11.58,-.27,;11.64,-1.8,;10.34,-2.62,;9.64,-7.72,;8.32,-6.94,;8.32,-5.41,;6.97,-7.71,;5.65,-6.93,;5.65,-5.39,;4.32,-4.6,;2.98,-5.37,;1.02,-5.44,;2.35,-6.19,;3.83,-5.79,;2.76,-7.69,;4.3,-7.69,;2.98,-6.91,;17.63,-7.69,;18.96,-6.92,;20.3,-7.67,;20.3,-9.22,;18.98,-9.99,;17.64,-9.23,)| Show InChI InChI=1S/C36H45N5O6/c1-36(19-27-20-37-29-11-7-6-10-28(27)29,40-35(45)47-33-25-15-22-14-23(17-25)18-26(33)16-22)34(44)38-21-30(24-8-4-3-5-9-24)39-31(42)12-13-32(43)41-46-2/h3-11,20,22-23,25-26,30,33,37H,12-19,21H2,1-2H3,(H,38,44)(H,39,42)(H,40,45)(H,41,43)/t22?,23?,25?,26?,30-,33?,36?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006884

((2-{2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-in...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCP(O)(O)=O)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25,(11.9,-7.66,;11.91,-6.12,;11.91,-4.58,;11.02,-3.31,;9.5,-3.55,;8.8,-2.17,;9.9,-1.08,;9.83,.46,;11.13,1.28,;12.51,.58,;12.56,-.96,;11.27,-1.8,;10.57,-6.89,;9.22,-6.12,;9.22,-4.58,;7.89,-6.88,;6.56,-6.1,;6.56,-4.56,;5.23,-3.78,;3.9,-4.53,;1.94,-4.6,;3.27,-5.37,;4.76,-4.97,;3.67,-6.87,;5.21,-6.87,;3.88,-6.07,;13.24,-6.89,;13.24,-8.43,;14.57,-6.12,;15.9,-6.88,;17.23,-6.1,;17.22,-4.56,;18.53,-3.78,;18.52,-2.24,;19.86,-4.53,;21.21,-3.74,;22.55,-4.5,;23.91,-5.23,;22.2,-6,;23.29,-3.13,;18.56,-6.87,;18.57,-8.41,;19.91,-9.17,;21.24,-8.38,;21.22,-6.84,;19.89,-6.09,)| Show InChI InChI=1S/C34H43N4O7P/c1-34(18-26-19-35-28-10-6-5-9-27(26)28,38-33(41)45-31-24-14-21-13-22(16-24)17-25(31)15-21)32(40)36-20-29(23-7-3-2-4-8-23)37-30(39)11-12-46(42,43)44/h2-10,19,21-22,24-25,29,31,35H,11-18,20H2,1H3,(H,36,40)(H,37,39)(H,38,41)(H2,42,43,44)/t21?,22?,24?,25?,29-,31?,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006888

((2-{2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-in...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCP(C)(O)=O)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25,(13.35,-7.14,;13.36,-5.6,;13.37,-4.06,;12.48,-2.78,;10.95,-3.02,;10.27,-1.64,;11.36,-.56,;11.3,.98,;12.58,1.81,;13.96,1.1,;14.03,-.44,;12.72,-1.27,;12.02,-6.35,;10.69,-5.58,;10.69,-4.04,;9.35,-6.35,;8.02,-5.57,;8.02,-4.02,;6.7,-3.25,;5.36,-4.01,;3.39,-4.07,;4.73,-4.84,;6.21,-4.44,;5.14,-6.33,;6.68,-6.33,;5.35,-5.55,;14.69,-6.37,;14.69,-7.91,;16.02,-5.58,;17.36,-6.35,;18.68,-5.57,;18.67,-4.04,;20,-3.25,;19.97,-1.71,;21.33,-4,;22.66,-3.22,;24,-3.97,;23.19,-5.29,;25.31,-4.77,;24.8,-2.64,;20.02,-6.33,;21.34,-5.56,;22.68,-6.31,;22.69,-7.86,;21.37,-8.64,;20.02,-7.87,)| Show InChI InChI=1S/C35H45N4O6P/c1-35(19-27-20-36-29-11-7-6-10-28(27)29,39-34(42)45-32-25-15-22-14-23(17-25)18-26(32)16-22)33(41)37-21-30(24-8-4-3-5-9-24)38-31(40)12-13-46(2,43)44/h3-11,20,22-23,25-26,30,32,36H,12-19,21H2,1-2H3,(H,37,41)(H,38,40)(H,39,42)(H,43,44)/t22?,23?,25?,26?,30-,32?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006879

(({2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-indo...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CP(O)(O)=O)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,(8.8,-9.36,;8.82,-7.82,;8.83,-6.28,;7.93,-5,;6.4,-5.23,;5.72,-3.86,;6.81,-2.78,;6.75,-1.24,;8.03,-.4,;9.41,-1.13,;9.48,-2.67,;8.17,-3.48,;7.47,-8.59,;6.14,-7.82,;6.14,-6.28,;4.81,-8.57,;3.48,-7.8,;3.48,-6.25,;2.15,-5.47,;.82,-6.24,;-1.16,-6.3,;.19,-7.07,;1.66,-6.66,;.59,-8.56,;2.13,-8.56,;.8,-7.78,;10.15,-8.59,;10.15,-10.13,;11.48,-7.82,;12.82,-8.57,;14.14,-7.8,;14.12,-6.26,;15.45,-5.47,;15.43,-3.93,;16.78,-6.23,;18.11,-5.44,;19.21,-6.53,;19.44,-4.67,;17.34,-4.11,;15.48,-8.56,;15.48,-10.1,;16.82,-10.86,;18.15,-10.09,;18.14,-8.54,;16.8,-7.78,)| Show InChI InChI=1S/C33H41N4O7P/c1-33(16-25-17-34-27-10-6-5-9-26(25)27,37-32(40)44-30-23-12-20-11-21(14-23)15-24(30)13-20)31(39)35-18-28(22-7-3-2-4-8-22)36-29(38)19-45(41,42)43/h2-10,17,20-21,23-24,28,30,34H,11-16,18-19H2,1H3,(H,35,39)(H,36,38)(H,37,40)(H2,41,42,43)/t20?,21?,23?,24?,28-,30?,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006886

(CHEMBL313756 | [1-[2-(3-Benzenesulfonylamino-propi...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCNS(=O)(=O)c1ccccc1)c1ccccc1 |wU:30.35,TLB:18:19:23:16.17.22,15:16:23:19.25.20,THB:20:21:16:19.18.25,20:19:16:21.23.22,18:17:23:19.25.20,(8.47,-8.68,;8.47,-7.15,;8.48,-5.61,;7.58,-4.32,;6.05,-4.55,;5.37,-3.19,;6.47,-2.11,;6.4,-.57,;7.69,.27,;9.07,-.45,;9.12,-1.99,;7.83,-2.81,;7.13,-7.9,;5.8,-7.13,;5.8,-5.6,;4.46,-7.9,;3.12,-7.12,;3.12,-5.58,;1.81,-4.79,;.48,-5.56,;-1.49,-5.62,;-.16,-6.38,;1.32,-5.98,;.24,-7.88,;1.79,-7.88,;.45,-7.1,;9.8,-7.9,;9.8,-9.45,;11.13,-7.13,;12.47,-7.9,;13.79,-7.12,;13.78,-5.58,;15.1,-4.79,;15.09,-3.26,;16.43,-5.55,;17.77,-4.76,;19.12,-5.54,;20.45,-4.74,;19.35,-3.64,;21.54,-3.64,;21.8,-5.51,;21.8,-7.06,;23.15,-7.83,;24.49,-7.04,;24.47,-5.48,;23.13,-4.72,;15.12,-7.88,;16.45,-7.11,;17.78,-7.86,;17.79,-9.4,;16.47,-10.18,;15.12,-9.42,)| Show InChI InChI=1S/C40H47N5O6S/c1-40(23-31-24-41-34-15-9-8-14-33(31)34,45-39(48)51-37-29-19-26-18-27(21-29)22-30(37)20-26)38(47)42-25-35(28-10-4-2-5-11-28)44-36(46)16-17-43-52(49,50)32-12-6-3-7-13-32/h2-15,24,26-27,29-30,35,37,41,43H,16-23,25H2,1H3,(H,42,47)(H,44,46)(H,45,48)/t26?,27?,29?,30?,35-,37?,40?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-B receptor by displacing [125I]bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006872
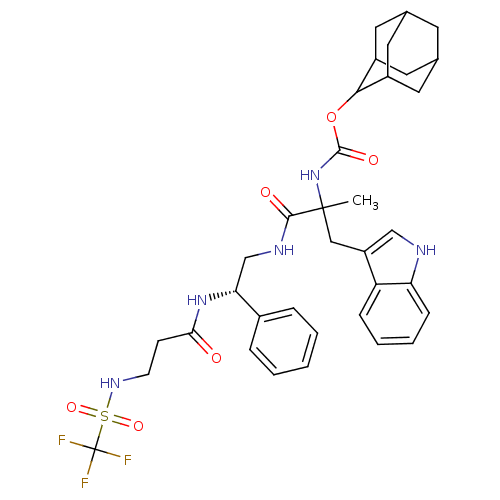
(CHEMBL86702 | {2-(1H-Indol-3-yl)-1-methyl-1-[2-phe...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCNS(=O)(=O)C(F)(F)F)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25,(9.64,-6.92,;9.64,-5.38,;9.66,-3.84,;8.75,-2.56,;7.23,-2.8,;6.55,-1.41,;7.64,-.34,;7.58,1.2,;8.87,2.03,;10.24,1.31,;10.3,-.22,;9.01,-1.04,;8.31,-6.14,;6.97,-5.38,;6.97,-3.83,;5.63,-6.13,;4.3,-5.35,;4.3,-3.81,;2.98,-3.03,;1.65,-3.8,;-.32,-3.85,;1.02,-4.63,;2.49,-4.22,;1.41,-6.12,;2.96,-6.12,;1.63,-5.33,;10.98,-6.15,;10.98,-7.69,;12.31,-5.38,;13.64,-6.13,;14.96,-5.35,;14.96,-3.82,;16.28,-3.03,;16.27,-1.48,;17.61,-3.78,;18.95,-3.01,;20.3,-3.77,;21.64,-2.98,;20.53,-1.88,;22.72,-1.88,;22.98,-3.75,;23,-5.3,;24.32,-2.96,;21.89,-4.85,;16.3,-6.12,;16.3,-7.65,;17.65,-8.42,;18.97,-7.63,;18.96,-6.09,;17.63,-5.34,)| Show InChI InChI=1S/C35H42F3N5O6S/c1-34(18-26-19-39-28-10-6-5-9-27(26)28,43-33(46)49-31-24-14-21-13-22(16-24)17-25(31)15-21)32(45)40-20-29(23-7-3-2-4-8-23)42-30(44)11-12-41-50(47,48)35(36,37)38/h2-10,19,21-22,24-25,29,31,39,41H,11-18,20H2,1H3,(H,40,45)(H,42,44)(H,43,46)/t21?,22?,24?,25?,29-,31?,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-B receptor by displacing [125I]bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006887

(CHEMBL86879 | [1-{2-[2-(2-Hydroxy-phenylsulfanyl)-...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CSc1ccccc1O)c1ccccc1 |TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25| Show InChI InChI=1S/C39H44N4O5S/c1-39(20-29-21-40-31-12-6-5-11-30(29)31,43-38(47)48-36-27-16-24-15-25(18-27)19-28(36)17-24)37(46)41-22-32(26-9-3-2-4-10-26)42-35(45)23-49-34-14-8-7-13-33(34)44/h2-14,21,24-25,27-28,32,36,40,44H,15-20,22-23H2,1H3,(H,41,46)(H,42,45)(H,43,47)/t24?,25?,27?,28?,32-,36?,39-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(MOUSE) | BDBM50006867
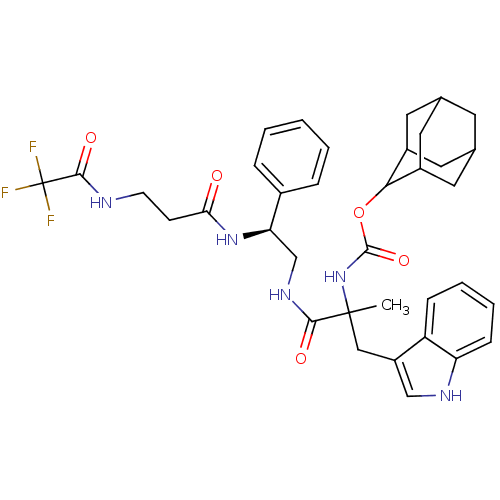
((2-(1H-Indol-3-yl)-1-methyl-1-{2-phenyl-2-[3-(2,2,...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCNC(=O)C(F)(F)F)c1ccccc1 |wU:30.35,TLB:25:24:22:19.18.20,THB:25:19:16.24.23:22,20:19:16:21.23.22,20:21:16:19.18.25,15:16:22:19.18.20,(9.64,-6.1,;9.64,-4.56,;9.66,-3.02,;8.75,-1.74,;7.23,-1.98,;6.55,-.59,;7.64,.48,;7.58,2.02,;8.87,2.85,;10.24,2.13,;10.3,.6,;9.01,-.22,;8.31,-5.32,;6.97,-4.56,;6.97,-3.01,;5.63,-5.31,;4.3,-4.53,;2.96,-5.3,;1.63,-4.51,;1.65,-2.98,;-.32,-3.03,;1.02,-3.81,;1.41,-5.3,;2.49,-3.4,;4.3,-2.98,;2.98,-2.21,;10.98,-5.33,;10.98,-6.87,;12.31,-4.56,;13.64,-5.31,;14.96,-4.53,;14.96,-3,;16.28,-2.21,;16.27,-.66,;17.61,-2.96,;18.95,-2.19,;20.3,-2.95,;21.64,-2.16,;21.62,-.62,;22.98,-2.93,;24.32,-2.14,;21.89,-4.03,;23,-4.48,;16.3,-5.3,;17.63,-4.52,;18.96,-5.27,;18.97,-6.82,;17.65,-7.6,;16.3,-6.83,)| Show InChI InChI=1S/C36H42F3N5O5/c1-35(18-26-19-41-28-10-6-5-9-27(26)28,44-34(48)49-31-24-14-21-13-22(16-24)17-25(31)15-21)32(46)42-20-29(23-7-3-2-4-8-23)43-30(45)11-12-40-33(47)36(37,38)39/h2-10,19,21-22,24-25,29,31,41H,11-18,20H2,1H3,(H,40,47)(H,42,46)(H,43,45)(H,44,48)/t21?,22?,24?,25?,29-,31?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type B receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the mouse cerebral cortex |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM81962

(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006886

(CHEMBL313756 | [1-[2-(3-Benzenesulfonylamino-propi...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCNS(=O)(=O)c1ccccc1)c1ccccc1 |wU:30.35,TLB:18:19:23:16.17.22,15:16:23:19.25.20,THB:20:21:16:19.18.25,20:19:16:21.23.22,18:17:23:19.25.20,(8.47,-8.68,;8.47,-7.15,;8.48,-5.61,;7.58,-4.32,;6.05,-4.55,;5.37,-3.19,;6.47,-2.11,;6.4,-.57,;7.69,.27,;9.07,-.45,;9.12,-1.99,;7.83,-2.81,;7.13,-7.9,;5.8,-7.13,;5.8,-5.6,;4.46,-7.9,;3.12,-7.12,;3.12,-5.58,;1.81,-4.79,;.48,-5.56,;-1.49,-5.62,;-.16,-6.38,;1.32,-5.98,;.24,-7.88,;1.79,-7.88,;.45,-7.1,;9.8,-7.9,;9.8,-9.45,;11.13,-7.13,;12.47,-7.9,;13.79,-7.12,;13.78,-5.58,;15.1,-4.79,;15.09,-3.26,;16.43,-5.55,;17.77,-4.76,;19.12,-5.54,;20.45,-4.74,;19.35,-3.64,;21.54,-3.64,;21.8,-5.51,;21.8,-7.06,;23.15,-7.83,;24.49,-7.04,;24.47,-5.48,;23.13,-4.72,;15.12,-7.88,;16.45,-7.11,;17.78,-7.86,;17.79,-9.4,;16.47,-10.18,;15.12,-9.42,)| Show InChI InChI=1S/C40H47N5O6S/c1-40(23-31-24-41-34-15-9-8-14-33(31)34,45-39(48)51-37-29-19-26-18-27(21-29)22-30(37)20-26)38(47)42-25-35(28-10-4-2-5-11-28)44-36(46)16-17-43-52(49,50)32-12-6-3-7-13-32/h2-15,24,26-27,29-30,35,37,41,43H,16-23,25H2,1H3,(H,42,47)(H,44,46)(H,45,48)/t26?,27?,29?,30?,35-,37?,40?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006877

(({2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-indo...)Show SMILES CCOP(O)(=O)CC(=O)N[C@H](CNC(=O)C(C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)c1ccccc1 |wU:10.9,TLB:30:31:33:36.37.35,THB:38:39:33:36.37.35,38:36:33:31.39.40,35:34:31:36.38.37,35:36:31:34.33.40,(26.18,-4.98,;24.95,-4.04,;23.53,-4.63,;22.31,-3.69,;23.53,-2.75,;21.37,-2.46,;20.98,-4.48,;19.65,-3.72,;19.63,-2.18,;18.32,-4.51,;18.34,-6.05,;17.02,-6.82,;15.68,-6.07,;14.35,-6.84,;14.35,-8.38,;13.02,-6.07,;13,-7.61,;13.03,-4.53,;12.13,-3.25,;10.6,-3.48,;9.92,-2.11,;11.01,-1.03,;10.95,.51,;12.23,1.35,;13.61,.62,;13.68,-.92,;12.37,-1.73,;11.67,-6.84,;10.34,-6.07,;10.34,-4.53,;9.01,-6.82,;7.68,-6.05,;7.68,-4.5,;6.35,-3.72,;5.02,-4.49,;3.04,-4.55,;4.39,-5.32,;5.86,-4.91,;4.79,-6.81,;6.33,-6.81,;5,-6.03,;19.68,-6.81,;21,-6.03,;22.34,-6.79,;22.35,-8.34,;21.02,-9.11,;19.68,-8.35,)| Show InChI InChI=1S/C35H45N4O7P/c1-3-45-47(43,44)21-31(40)38-30(24-9-5-4-6-10-24)20-37-33(41)35(2,18-27-19-36-29-12-8-7-11-28(27)29)39-34(42)46-32-25-14-22-13-23(16-25)17-26(32)15-22/h4-12,19,22-23,25-26,30,32,36H,3,13-18,20-21H2,1-2H3,(H,37,41)(H,38,40)(H,39,42)(H,43,44)/t22?,23?,25?,26?,30-,32?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006887

(CHEMBL86879 | [1-{2-[2-(2-Hydroxy-phenylsulfanyl)-...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CSc1ccccc1O)c1ccccc1 |TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25| Show InChI InChI=1S/C39H44N4O5S/c1-39(20-29-21-40-31-12-6-5-11-30(29)31,43-38(47)48-36-27-16-24-15-25(18-27)19-28(36)17-24)37(46)41-22-32(26-9-3-2-4-10-26)42-35(45)23-49-34-14-8-7-13-33(34)44/h2-14,21,24-25,27-28,32,36,40,44H,15-20,22-23H2,1H3,(H,41,46)(H,42,45)(H,43,47)/t24?,25?,27?,28?,32-,36?,39-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50024321

(3-{2-[2-(3-tert-Butoxycarbonylamino-propionylamino...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H49N7O9S/c1-37(2,3)53-36(52)39-16-14-30(45)41-28(19-23-21-40-25-13-9-8-12-24(23)25)34(50)42-26(15-17-54-4)33(49)44-29(20-31(46)47)35(51)43-27(32(38)48)18-22-10-6-5-7-11-22/h5-13,21,26-29,40H,14-20H2,1-4H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47)/t26-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50455601

(CHEMBL2112331)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CSc1c[nH]nn1)c1ccccc1 |TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25| Show InChI InChI=1S/C35H41N7O4S/c1-35(16-26-17-36-28-10-6-5-9-27(26)28,40-34(45)46-32-24-12-21-11-22(14-24)15-25(32)13-21)33(44)37-18-29(23-7-3-2-4-8-23)39-30(43)20-47-31-19-38-42-41-31/h2-10,17,19,21-22,24-25,29,32,36H,11-16,18,20H2,1H3,(H,37,44)(H,39,43)(H,40,45)(H,38,41,42)/t21?,22?,24?,25?,29-,32?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50455598

(CHEMBL2112335)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCn1nn[nH]c1=S)c1ccccc1 |TLB:25:24:22:19.18.20,THB:15:16:22:19.18.20,20:19:16:21.23.22,20:21:16:19.18.25,25:19:16.24.23:22| Show InChI InChI=1S/C35H42N8O4S/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(46)47-31-24-14-21-13-22(16-24)17-25(31)15-21)32(45)37-20-29(23-7-3-2-4-8-23)38-30(44)11-12-43-33(48)40-41-42-43/h2-10,19,21-22,24-25,29,31,36H,11-18,20H2,1H3,(H,37,45)(H,38,44)(H,39,46)(H,40,42,48)/t21?,22?,24?,25?,29-,31?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50455599

(CHEMBL2112333)Show SMILES [H][C@](CNC(=O)[C@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)CSc1n[nH]c(=O)[nH]1)c1ccccc1 |wU:1.36,wD:6.7,1.0,TLB:21:22:24:27.28.26,THB:26:25:22:27.29.28,26:27:22:25.24.31,29:30:24:27.28.26,29:27:24:22.30.31,(14.15,-7.57,;12.83,-8.34,;11.5,-9.12,;10.17,-8.37,;8.85,-9.14,;8.85,-10.68,;7.52,-8.37,;7.52,-9.91,;7.52,-6.84,;6.63,-5.56,;5.11,-5.79,;4.43,-4.41,;5.51,-3.33,;5.46,-1.8,;6.74,-.97,;8.11,-1.69,;8.18,-3.22,;6.88,-4.04,;6.18,-9.13,;4.85,-8.37,;4.85,-6.82,;3.51,-9.12,;2.19,-8.34,;2.19,-6.8,;.87,-6.03,;-.47,-6.79,;-2.44,-6.85,;-1.1,-7.62,;.87,-7.93,;-.69,-9.11,;.84,-9.11,;-.48,-8.32,;12.82,-6.81,;14.15,-6.03,;14.13,-4.48,;15.49,-6.77,;16.82,-6,;18.16,-6.77,;18.34,-8.29,;19.86,-8.6,;20.61,-7.26,;22.15,-7.08,;19.58,-6.12,;14.17,-9.11,;14.17,-10.64,;15.51,-11.41,;16.84,-10.63,;16.82,-9.09,;15.49,-8.32,)| Show InChI InChI=1S/C35H41N7O5S/c1-35(16-25-17-36-27-10-6-5-9-26(25)27,40-34(46)47-30-23-12-20-11-21(14-23)15-24(30)13-20)31(44)37-18-28(22-7-3-2-4-8-22)38-29(43)19-48-33-39-32(45)41-42-33/h2-10,17,20-21,23-24,28,30,36H,11-16,18-19H2,1H3,(H,37,44)(H,38,43)(H,40,46)(H2,39,41,42,45)/t20?,21?,23?,24?,28-,30?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50455600

(CHEMBL2112330)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CSc1nc[nH]n1)c1ccccc1 |TLB:15:16:23:19.25.20,18:19:16.17.22:23,THB:20:19:16:21.22.23,20:21:16:19.18.25,18:17:23:19.25.20| Show InChI InChI=1S/C35H41N7O4S/c1-35(16-26-17-36-28-10-6-5-9-27(26)28,41-34(45)46-31-24-12-21-11-22(14-24)15-25(31)13-21)32(44)37-18-29(23-7-3-2-4-8-23)40-30(43)19-47-33-38-20-39-42-33/h2-10,17,20-22,24-25,29,31,36H,11-16,18-19H2,1H3,(H,37,44)(H,40,43)(H,41,45)(H,38,39,42)/t21?,22?,24?,25?,29-,31?,35+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006872

(CHEMBL86702 | {2-(1H-Indol-3-yl)-1-methyl-1-[2-phe...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCNS(=O)(=O)C(F)(F)F)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25,(9.64,-6.92,;9.64,-5.38,;9.66,-3.84,;8.75,-2.56,;7.23,-2.8,;6.55,-1.41,;7.64,-.34,;7.58,1.2,;8.87,2.03,;10.24,1.31,;10.3,-.22,;9.01,-1.04,;8.31,-6.14,;6.97,-5.38,;6.97,-3.83,;5.63,-6.13,;4.3,-5.35,;4.3,-3.81,;2.98,-3.03,;1.65,-3.8,;-.32,-3.85,;1.02,-4.63,;2.49,-4.22,;1.41,-6.12,;2.96,-6.12,;1.63,-5.33,;10.98,-6.15,;10.98,-7.69,;12.31,-5.38,;13.64,-6.13,;14.96,-5.35,;14.96,-3.82,;16.28,-3.03,;16.27,-1.48,;17.61,-3.78,;18.95,-3.01,;20.3,-3.77,;21.64,-2.98,;20.53,-1.88,;22.72,-1.88,;22.98,-3.75,;23,-5.3,;24.32,-2.96,;21.89,-4.85,;16.3,-6.12,;16.3,-7.65,;17.65,-8.42,;18.97,-7.63,;18.96,-6.09,;17.63,-5.34,)| Show InChI InChI=1S/C35H42F3N5O6S/c1-34(18-26-19-39-28-10-6-5-9-27(26)28,43-33(46)49-31-24-14-21-13-22(16-24)17-25(31)15-21)32(45)40-20-29(23-7-3-2-4-8-23)42-30(44)11-12-41-50(47,48)35(36,37)38/h2-10,19,21-22,24-25,29,31,39,41H,11-18,20H2,1H3,(H,40,45)(H,42,44)(H,43,46)/t21?,22?,24?,25?,29-,31?,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of CCK-A receptor by displacing [125I]-Bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006867

((2-(1H-Indol-3-yl)-1-methyl-1-{2-phenyl-2-[3-(2,2,...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCNC(=O)C(F)(F)F)c1ccccc1 |wU:30.35,TLB:25:24:22:19.18.20,THB:25:19:16.24.23:22,20:19:16:21.23.22,20:21:16:19.18.25,15:16:22:19.18.20,(9.64,-6.1,;9.64,-4.56,;9.66,-3.02,;8.75,-1.74,;7.23,-1.98,;6.55,-.59,;7.64,.48,;7.58,2.02,;8.87,2.85,;10.24,2.13,;10.3,.6,;9.01,-.22,;8.31,-5.32,;6.97,-4.56,;6.97,-3.01,;5.63,-5.31,;4.3,-4.53,;2.96,-5.3,;1.63,-4.51,;1.65,-2.98,;-.32,-3.03,;1.02,-3.81,;1.41,-5.3,;2.49,-3.4,;4.3,-2.98,;2.98,-2.21,;10.98,-5.33,;10.98,-6.87,;12.31,-4.56,;13.64,-5.31,;14.96,-4.53,;14.96,-3,;16.28,-2.21,;16.27,-.66,;17.61,-2.96,;18.95,-2.19,;20.3,-2.95,;21.64,-2.16,;21.62,-.62,;22.98,-2.93,;24.32,-2.14,;21.89,-4.03,;23,-4.48,;16.3,-5.3,;17.63,-4.52,;18.96,-5.27,;18.97,-6.82,;17.65,-7.6,;16.3,-6.83,)| Show InChI InChI=1S/C36H42F3N5O5/c1-35(18-26-19-41-28-10-6-5-9-27(26)28,44-34(48)49-31-24-14-21-13-22(16-24)17-25(31)15-21)32(46)42-20-29(23-7-3-2-4-8-23)43-30(45)11-12-40-33(47)36(37,38)39/h2-10,19,21-22,24-25,29,31,41H,11-18,20H2,1H3,(H,40,47)(H,42,46)(H,43,45)(H,44,48)/t21?,22?,24?,25?,29-,31?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006868

((2-(1H-Indol-3-yl)-1-methyl-1-{2-phenyl-2-[3-(2H-[...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCSc1nnc[nH]1)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25,(12.99,-8.61,;12.99,-7.06,;12.99,-5.53,;12.1,-4.25,;10.58,-4.49,;9.9,-3.1,;10.98,-2.02,;10.92,-.49,;12.21,.34,;13.58,-.38,;13.65,-1.91,;12.35,-2.73,;11.65,-7.83,;10.32,-7.06,;10.32,-5.52,;8.98,-7.81,;7.65,-7.04,;7.65,-5.49,;6.33,-4.72,;4.99,-5.49,;3.02,-5.54,;4.34,-6.31,;5.84,-5.91,;4.77,-7.81,;6.31,-7.81,;4.98,-7.02,;14.32,-7.84,;14.32,-9.38,;15.64,-7.06,;16.98,-7.81,;18.31,-7.04,;18.29,-5.51,;19.63,-4.72,;19.6,-3.17,;20.96,-5.47,;22.3,-4.7,;23.65,-5.45,;25,-6.22,;25.18,-7.74,;26.68,-8.05,;27.45,-6.71,;26.4,-5.56,;19.64,-7.81,;19.65,-9.34,;20.99,-10.11,;22.32,-9.33,;22.3,-7.78,;20.96,-7.03,)| Show InChI InChI=1S/C36H43N7O4S/c1-36(18-27-19-37-29-10-6-5-9-28(27)29,42-35(46)47-32-25-14-22-13-23(16-25)17-26(32)15-22)33(45)38-20-30(24-7-3-2-4-8-24)41-31(44)11-12-48-34-39-21-40-43-34/h2-10,19,21-23,25-26,30,32,37H,11-18,20H2,1H3,(H,38,45)(H,41,44)(H,42,46)(H,39,40,43)/t22?,23?,25?,26?,30-,32?,36?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50455596

(CHEMBL2112336)Show SMILES [H][C@](CNC(=O)[C@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)C[S+]([O-])c1nc[nH]n1)c1ccccc1 |wU:1.36,wD:6.7,1.0,TLB:21:22:24:27.28.26,THB:26:25:22:27.29.28,26:27:22:25.24.31,29:30:24:27.28.26,29:27:24:22.30.31,(5.59,-7.16,;4.27,-7.93,;2.94,-8.7,;1.6,-7.94,;.28,-8.71,;.28,-10.24,;-1.05,-7.94,;-1.06,-9.47,;-1.04,-6.39,;-1.93,-5.12,;-3.45,-5.35,;-4.13,-3.97,;-3.05,-2.89,;-3.1,-1.35,;-1.81,-.52,;-.44,-1.24,;-.37,-2.78,;-1.67,-3.6,;-2.39,-8.69,;-3.72,-7.93,;-3.71,-6.39,;-5.07,-8.67,;-6.39,-7.9,;-6.39,-6.36,;-7.71,-5.57,;-9.05,-6.32,;-11.02,-6.39,;-9.68,-7.16,;-7.82,-7.41,;-9.28,-8.64,;-7.74,-8.65,;-9.07,-7.87,;4.26,-6.39,;5.6,-5.61,;5.58,-4.07,;6.93,-6.37,;8.27,-5.59,;8.27,-4.05,;9.61,-6.36,;9.79,-7.88,;11.3,-8.21,;12.07,-6.85,;11.03,-5.72,;5.6,-8.69,;5.61,-10.24,;6.94,-11.01,;8.28,-10.22,;8.26,-8.67,;6.93,-7.92,)| Show InChI InChI=1S/C35H41N7O5S/c1-35(16-26-17-36-28-10-6-5-9-27(26)28,41-34(45)47-31-24-12-21-11-22(14-24)15-25(31)13-21)32(44)37-18-29(23-7-3-2-4-8-23)40-30(43)19-48(46)33-38-20-39-42-33/h2-10,17,20-22,24-25,29,31,36H,11-16,18-19H2,1H3,(H,37,44)(H,40,43)(H,41,45)(H,38,39,42)/t21?,22?,24?,25?,29-,31?,35+,48?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006881

(CHEMBL88090 | {2-(1H-Indol-3-yl)-1-methyl-1-[2-phe...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCc1nnn[nH]1)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25,(10.08,-15.84,;10.09,-14.29,;10.1,-12.76,;9.2,-11.48,;7.68,-11.72,;7,-10.34,;8.09,-9.26,;8.02,-7.73,;9.31,-6.89,;10.69,-7.61,;10.75,-9.14,;9.45,-9.96,;8.76,-15.06,;7.42,-14.29,;7.42,-12.75,;6.07,-15.04,;4.75,-14.27,;4.75,-12.73,;3.43,-11.95,;2.09,-12.72,;.13,-12.77,;1.46,-13.55,;2.95,-13.14,;1.87,-15.04,;3.4,-15.04,;2.08,-14.25,;11.41,-15.07,;11.41,-16.61,;12.75,-14.29,;14.08,-15.04,;15.4,-14.27,;15.39,-12.74,;16.72,-11.95,;16.71,-10.41,;18.06,-12.7,;19.39,-11.93,;20.74,-12.69,;22.28,-12.65,;22.8,-14.11,;21.58,-15.04,;20.29,-14.17,;16.73,-15.04,;16.75,-16.57,;18.09,-17.34,;19.42,-16.56,;19.4,-15.01,;18.07,-14.26,)| Show InChI InChI=1S/C35H42N8O4/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(46)47-32-24-14-21-13-22(16-24)17-25(32)15-21)33(45)37-20-29(23-7-3-2-4-8-23)38-31(44)12-11-30-40-42-43-41-30/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,45)(H,38,44)(H,39,46)(H,40,41,42,43)/t21?,22?,24?,25?,29-,32?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50455595

(CHEMBL2112338)Show SMILES C[C@@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCS([O-])(=O)=O)c1ccccc1 |TLB:18:19:23:16.17.22,15:16:23:19.25.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,18:17:23:19.25.20| Show InChI InChI=1S/C34H42N4O7S.Na/c1-34(18-26-19-35-28-10-6-5-9-27(26)28,38-33(41)45-31-24-14-21-13-22(16-24)17-25(31)15-21)32(40)36-20-29(23-7-3-2-4-8-23)37-30(39)11-12-46(42,43)44;/h2-10,19,21-22,24-25,29,31,35H,11-18,20H2,1H3,(H,36,40)(H,37,39)(H,38,41)(H,42,43,44);/q;+1/p-1/t21?,22?,24?,25?,29-,31?,34+;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006883

(CHEMBL313499 | [1-[2-(3-Hydroxycarbamoyl-propionyl...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCC(=O)NO)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,(9.29,-10.11,;9.29,-8.57,;9.29,-7.02,;8.4,-5.75,;6.88,-5.98,;6.2,-4.6,;7.28,-3.53,;7.22,-1.98,;8.51,-1.15,;9.88,-1.86,;9.95,-3.41,;8.65,-4.23,;7.95,-9.32,;6.62,-8.56,;6.62,-7.02,;5.28,-9.31,;3.95,-8.54,;3.95,-7,;2.63,-6.21,;1.29,-6.97,;-.68,-7.04,;.66,-7.81,;2.13,-7.4,;1.06,-9.29,;2.6,-9.29,;1.27,-8.52,;10.62,-9.33,;10.62,-10.87,;11.94,-8.56,;13.28,-9.32,;14.61,-8.54,;14.6,-7,;15.93,-6.21,;15.91,-4.67,;17.27,-6.97,;18.61,-6.19,;19.94,-6.95,;19.97,-8.5,;21.28,-6.17,;22.63,-6.93,;15.95,-9.29,;15.96,-10.84,;17.29,-11.61,;18.63,-10.82,;18.61,-9.27,;17.27,-8.52,)| Show InChI InChI=1S/C35H43N5O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)46-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(41)11-12-31(42)40-45/h2-10,19,21-22,24-25,29,32,36,45H,11-18,20H2,1H3,(H,37,43)(H,38,41)(H,39,44)(H,40,42)/t21?,22?,24?,25?,29-,32?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006885

((2-(1H-Indol-3-yl)-1-methyl-1-{2-phenyl-2-[3-(5H-t...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCC(=O)Nc1nnn[nH]1)c1ccccc1 |wU:30.35,TLB:25:24:22:19.18.20,THB:15:16:22:19.18.20,20:19:16:21.22.23,20:21:16:19.18.25,25:19:22:16.24.23,(5.37,-5.89,;5.39,-4.34,;5.4,-2.81,;4.5,-1.53,;2.98,-1.76,;2.3,-.38,;3.38,.69,;3.31,2.23,;4.6,3.06,;5.98,2.34,;6.04,.81,;4.74,-.01,;4.05,-5.11,;2.72,-4.33,;2.72,-2.8,;1.37,-5.09,;.05,-4.32,;.05,-2.77,;-1.76,-3.17,;-3.25,-3.58,;-4.58,-2.82,;-2.62,-2.76,;-1.28,-2,;-2.62,-4.29,;-1.3,-5.08,;-2.83,-5.08,;6.71,-5.11,;6.71,-6.65,;8.04,-4.33,;9.38,-5.09,;10.69,-4.32,;10.68,-2.79,;12.02,-2,;12,-.45,;13.35,-2.75,;14.69,-1.98,;16.03,-2.74,;16.05,-4.29,;17.37,-1.95,;18.7,-2.72,;20.12,-2.07,;21.16,-3.21,;20.4,-4.55,;18.89,-4.25,;12.02,-5.08,;12.04,-6.61,;13.38,-7.39,;14.71,-6.61,;14.7,-5.06,;13.36,-4.31,)| Show InChI InChI=1S/C36H43N9O5/c1-36(18-26-19-37-28-10-6-5-9-27(26)28,41-35(49)50-32-24-14-21-13-22(16-24)17-25(32)15-21)33(48)38-20-29(23-7-3-2-4-8-23)39-30(46)11-12-31(47)40-34-42-44-45-43-34/h2-10,19,21-22,24-25,29,32,37H,11-18,20H2,1H3,(H,38,48)(H,39,46)(H,41,49)(H2,40,42,43,44,45,47)/t21?,22?,24?,25?,29-,32?,36?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006882

(CHEMBL312894 | {2-(1H-Indol-3-yl)-1-[2-(3-methoxyc...)Show SMILES CONC(=O)CCC(=O)N[C@H](CNC(=O)C(C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)c1ccccc1 |wU:10.9,TLB:30:31:33:36.37.35,THB:38:39:33:36.37.35,38:36:33:31.39.40,35:34:31:36.38.37,35:36:31:34.33.40,(24.33,-6.87,;24.3,-5.33,;22.97,-4.55,;21.63,-5.35,;21.65,-6.9,;20.29,-4.58,;18.95,-5.36,;17.62,-4.6,;17.6,-3.07,;16.28,-5.4,;16.29,-6.93,;14.98,-7.71,;13.64,-6.94,;12.31,-7.72,;12.31,-9.26,;10.99,-6.96,;10.97,-8.49,;11,-5.42,;10.1,-4.13,;8.57,-4.37,;7.9,-3,;8.98,-1.92,;8.91,-.38,;10.2,.46,;11.58,-.27,;11.64,-1.8,;10.34,-2.62,;9.64,-7.72,;8.32,-6.94,;8.32,-5.41,;6.97,-7.71,;5.65,-6.93,;5.65,-5.39,;4.32,-4.6,;2.98,-5.37,;1.02,-5.44,;2.35,-6.19,;3.83,-5.79,;2.76,-7.69,;4.3,-7.69,;2.98,-6.91,;17.63,-7.69,;18.96,-6.92,;20.3,-7.67,;20.3,-9.22,;18.98,-9.99,;17.64,-9.23,)| Show InChI InChI=1S/C36H45N5O6/c1-36(19-27-20-37-29-11-7-6-10-28(27)29,40-35(45)47-33-25-15-22-14-23(17-25)18-26(33)16-22)34(44)38-21-30(24-8-4-3-5-9-24)39-31(42)12-13-32(43)41-46-2/h3-11,20,22-23,25-26,30,33,37H,12-19,21H2,1-2H3,(H,38,44)(H,39,42)(H,40,45)(H,41,43)/t22?,23?,25?,26?,30-,33?,36?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50455597

(CHEMBL2112334)Show SMILES [H][C@](CNC(=O)[C@](C)(Cc1c[nH]c2ccccc12)NC(=O)OC1C2CC3CC(C2)CC1C3)(NC(=O)Cc1cc(O)no1)c1ccccc1 |wU:1.36,wD:6.7,1.0,TLB:21:22:29:25.31.26,24:25:22.23.28:29,THB:26:25:22:27.28.29,26:27:22:25.24.31,24:23:29:25.31.26,(18.39,-8.83,;17.06,-9.61,;15.75,-10.38,;14.41,-9.63,;13.08,-10.4,;13.08,-11.93,;11.76,-9.63,;11.74,-11.17,;11.76,-8.09,;10.87,-6.81,;9.34,-7.05,;8.66,-5.68,;9.75,-4.6,;9.68,-3.05,;10.97,-2.22,;12.35,-2.94,;12.41,-4.48,;11.11,-5.3,;10.42,-10.38,;9.09,-9.63,;9.09,-8.09,;7.74,-10.37,;6.42,-9.61,;5.07,-10.36,;3.54,-10.36,;3.12,-8.88,;1.79,-8.11,;3.75,-8.04,;3.75,-9.59,;5.09,-7.28,;6.42,-8.06,;4.61,-8.74,;17.05,-8.06,;18.38,-7.28,;18.37,-5.75,;19.7,-8.04,;21.05,-7.26,;21.2,-5.71,;22.7,-5.39,;23.33,-3.97,;23.5,-6.71,;22.47,-7.85,;18.39,-10.36,;19.73,-9.59,;21.06,-10.34,;21.08,-11.88,;19.75,-12.67,;18.41,-11.9,)| Show InChI InChI=1S/C36H41N5O6/c1-36(18-26-19-37-29-10-6-5-9-28(26)29,40-35(45)46-33-24-12-21-11-22(14-24)15-25(33)13-21)34(44)38-20-30(23-7-3-2-4-8-23)39-31(42)16-27-17-32(43)41-47-27/h2-10,17,19,21-22,24-25,30,33,37H,11-16,18,20H2,1H3,(H,38,44)(H,39,42)(H,40,45)(H,41,43)/t21?,22?,24?,25?,30-,33?,36+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of CCK-A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006889

(({2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-indo...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CP(C)(O)=O)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,(11.35,-13.19,;11.36,-11.65,;11.37,-10.11,;10.48,-8.83,;8.94,-9.06,;8.27,-7.68,;9.36,-6.61,;9.29,-5.07,;10.58,-4.23,;11.95,-4.95,;12.02,-6.49,;10.72,-7.31,;10.02,-12.41,;8.69,-11.64,;8.69,-10.1,;7.35,-12.4,;6.02,-11.63,;6.02,-10.08,;4.7,-9.29,;3.36,-10.06,;1.38,-10.13,;2.73,-10.9,;4.21,-10.48,;3.13,-12.39,;4.67,-12.39,;3.34,-11.6,;12.69,-12.42,;12.69,-13.96,;14.02,-11.64,;15.36,-12.4,;16.68,-11.63,;16.67,-10.09,;18,-9.29,;17.97,-7.75,;19.33,-10.06,;20.66,-9.27,;21.59,-10.51,;21.87,-8.33,;19.72,-8.03,;18.02,-12.39,;18.02,-13.93,;19.37,-14.68,;20.7,-13.91,;20.68,-12.37,;19.34,-11.6,)| Show InChI InChI=1S/C34H43N4O6P/c1-34(17-26-18-35-28-11-7-6-10-27(26)28,38-33(41)44-31-24-13-21-12-22(15-24)16-25(31)14-21)32(40)36-19-29(23-8-4-3-5-9-23)37-30(39)20-45(2,42)43/h3-11,18,21-22,24-25,29,31,35H,12-17,19-20H2,1-2H3,(H,36,40)(H,37,39)(H,38,41)(H,42,43)/t21?,22?,24?,25?,29-,31?,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006884

((2-{2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-in...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCP(O)(O)=O)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25,(11.9,-7.66,;11.91,-6.12,;11.91,-4.58,;11.02,-3.31,;9.5,-3.55,;8.8,-2.17,;9.9,-1.08,;9.83,.46,;11.13,1.28,;12.51,.58,;12.56,-.96,;11.27,-1.8,;10.57,-6.89,;9.22,-6.12,;9.22,-4.58,;7.89,-6.88,;6.56,-6.1,;6.56,-4.56,;5.23,-3.78,;3.9,-4.53,;1.94,-4.6,;3.27,-5.37,;4.76,-4.97,;3.67,-6.87,;5.21,-6.87,;3.88,-6.07,;13.24,-6.89,;13.24,-8.43,;14.57,-6.12,;15.9,-6.88,;17.23,-6.1,;17.22,-4.56,;18.53,-3.78,;18.52,-2.24,;19.86,-4.53,;21.21,-3.74,;22.55,-4.5,;23.91,-5.23,;22.2,-6,;23.29,-3.13,;18.56,-6.87,;18.57,-8.41,;19.91,-9.17,;21.24,-8.38,;21.22,-6.84,;19.89,-6.09,)| Show InChI InChI=1S/C34H43N4O7P/c1-34(18-26-19-35-28-10-6-5-9-27(26)28,38-33(41)45-31-24-14-21-13-22(16-24)17-25(31)15-21)32(40)36-20-29(23-7-3-2-4-8-23)37-30(39)11-12-46(42,43)44/h2-10,19,21-22,24-25,29,31,35H,11-18,20H2,1H3,(H,36,40)(H,37,39)(H,38,41)(H2,42,43,44)/t21?,22?,24?,25?,29-,31?,34?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006888

((2-{2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-in...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCP(C)(O)=O)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:20:19:16:21.23.22,20:21:16:19.18.25,23:24:18:21.22.20,23:21:18:16.24.25,(13.35,-7.14,;13.36,-5.6,;13.37,-4.06,;12.48,-2.78,;10.95,-3.02,;10.27,-1.64,;11.36,-.56,;11.3,.98,;12.58,1.81,;13.96,1.1,;14.03,-.44,;12.72,-1.27,;12.02,-6.35,;10.69,-5.58,;10.69,-4.04,;9.35,-6.35,;8.02,-5.57,;8.02,-4.02,;6.7,-3.25,;5.36,-4.01,;3.39,-4.07,;4.73,-4.84,;6.21,-4.44,;5.14,-6.33,;6.68,-6.33,;5.35,-5.55,;14.69,-6.37,;14.69,-7.91,;16.02,-5.58,;17.36,-6.35,;18.68,-5.57,;18.67,-4.04,;20,-3.25,;19.97,-1.71,;21.33,-4,;22.66,-3.22,;24,-3.97,;23.19,-5.29,;25.31,-4.77,;24.8,-2.64,;20.02,-6.33,;21.34,-5.56,;22.68,-6.31,;22.69,-7.86,;21.37,-8.64,;20.02,-7.87,)| Show InChI InChI=1S/C35H45N4O6P/c1-35(19-27-20-36-29-11-7-6-10-28(27)29,39-34(42)45-32-25-15-22-14-23(17-25)18-26(32)16-22)33(41)37-21-30(24-8-4-3-5-9-24)38-31(40)12-13-46(2,43)44/h3-11,20,22-23,25-26,30,32,36H,12-19,21H2,1-2H3,(H,37,41)(H,38,40)(H,39,42)(H,43,44)/t22?,23?,25?,26?,30-,32?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006875

(CHEMBL263969 | N-{(S)-2-[(R)-2-(Adamantan-2-yloxyc...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CCC(O)=O)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,(5.02,-9.99,;5.02,-8.45,;5.04,-6.91,;4.14,-5.65,;2.62,-5.88,;1.94,-4.5,;3.02,-3.41,;2.96,-1.87,;4.25,-1.05,;5.63,-1.76,;5.69,-3.3,;4.39,-4.13,;3.69,-9.22,;2.36,-8.45,;2.36,-6.91,;1.01,-9.22,;-.32,-8.43,;-.32,-6.89,;-1.63,-6.11,;-2.98,-6.87,;-4.94,-6.94,;-3.61,-7.71,;-2.12,-7.3,;-3.2,-9.2,;-1.66,-9.2,;-2.99,-8.41,;6.35,-9.22,;6.35,-10.76,;7.68,-8.45,;9.03,-9.22,;10.34,-8.43,;10.34,-6.89,;11.67,-6.11,;11.65,-4.57,;13,-6.87,;14.33,-6.07,;15.66,-6.84,;16.99,-6.05,;15.69,-8.38,;11.69,-9.2,;11.7,-10.74,;13.03,-11.5,;14.36,-10.72,;14.35,-9.18,;13,-8.41,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t21?,22?,24?,25?,29-,32?,35?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(RAT) | BDBM50006879

(({2-[2-(Adamantan-2-yloxycarbonylamino)-3-(1H-indo...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NC[C@@H](NC(=O)CP(O)(O)=O)c1ccccc1 |wU:30.35,TLB:15:16:18:21.22.20,THB:23:24:18:21.22.20,23:21:18:16.24.25,20:19:16:21.23.22,20:21:16:19.18.25,(8.8,-9.36,;8.82,-7.82,;8.83,-6.28,;7.93,-5,;6.4,-5.23,;5.72,-3.86,;6.81,-2.78,;6.75,-1.24,;8.03,-.4,;9.41,-1.13,;9.48,-2.67,;8.17,-3.48,;7.47,-8.59,;6.14,-7.82,;6.14,-6.28,;4.81,-8.57,;3.48,-7.8,;3.48,-6.25,;2.15,-5.47,;.82,-6.24,;-1.16,-6.3,;.19,-7.07,;1.66,-6.66,;.59,-8.56,;2.13,-8.56,;.8,-7.78,;10.15,-8.59,;10.15,-10.13,;11.48,-7.82,;12.82,-8.57,;14.14,-7.8,;14.12,-6.26,;15.45,-5.47,;15.43,-3.93,;16.78,-6.23,;18.11,-5.44,;19.21,-6.53,;19.44,-4.67,;17.34,-4.11,;15.48,-8.56,;15.48,-10.1,;16.82,-10.86,;18.15,-10.09,;18.14,-8.54,;16.8,-7.78,)| Show InChI InChI=1S/C33H41N4O7P/c1-33(16-25-17-34-27-10-6-5-9-26(25)27,37-32(40)44-30-23-12-20-11-21(14-23)15-24(30)13-20)31(39)35-18-28(22-7-3-2-4-8-22)36-29(38)19-45(41,42)43/h2-10,17,20-21,23-24,28,30,34H,11-16,18-19H2,1H3,(H,35,39)(H,36,38)(H,37,40)(H2,41,42,43)/t20?,21?,23?,24?,28-,30?,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Evaluated for inhibition of cholecystokinin type A receptor by displacing [125I]bolton hunter CCK-8 radioligand in the rat pancreas |
J Med Chem 35: 2573-81 (1992)
BindingDB Entry DOI: 10.7270/Q2TT4RJ4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































