

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180049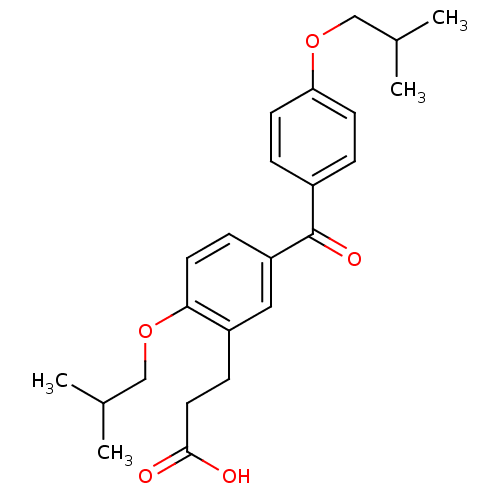 (3-[2-isobutoxy-5-(4-isobutoxybenzoyl)phenyl]propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of the expression of AP1-luciferase by TPA-stimulated NIH3T3 cells | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180047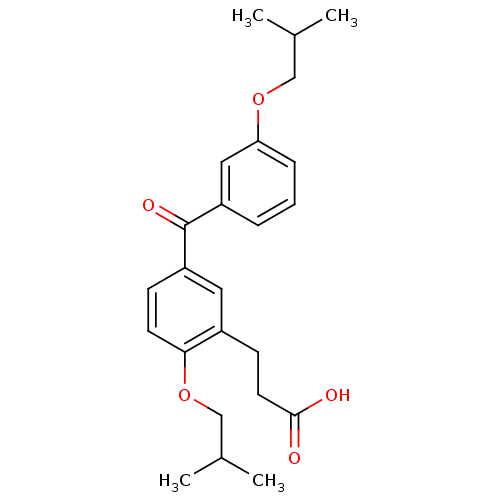 (3-[2-isobutoxy-5-(3-isobutoxybenzoyl)phenyl]propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of the expression of AP1-luciferase by TPA-stimulated NIH3T3 cells | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180051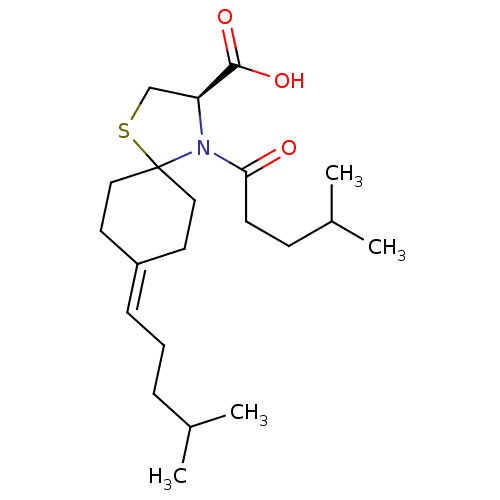 ((R)-4-(4-methylpentanoyl)-8-(4-methylpentylidene)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of the expression of AP1-luciferase by TPA-stimulated NIH3T3 cells | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180048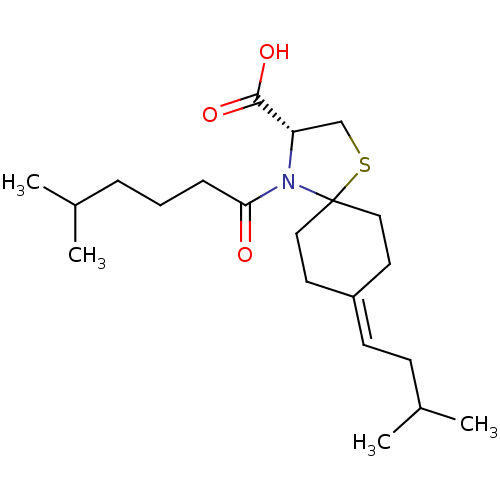 ((R)-8-(3-methylbutylidene)-4-(5-methylhexanoyl)-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of the expression of AP1-luciferase by TPA-stimulated NIH3T3 cells | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180050 (3-[2-benzyloxy-5-(4-isobutoxybenzoyl)phenyl]propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180048 ((R)-8-(3-methylbutylidene)-4-(5-methylhexanoyl)-1-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180047 (3-[2-isobutoxy-5-(3-isobutoxybenzoyl)phenyl]propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180049 (3-[2-isobutoxy-5-(4-isobutoxybenzoyl)phenyl]propio...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180051 ((R)-4-(4-methylpentanoyl)-8-(4-methylpentylidene)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180052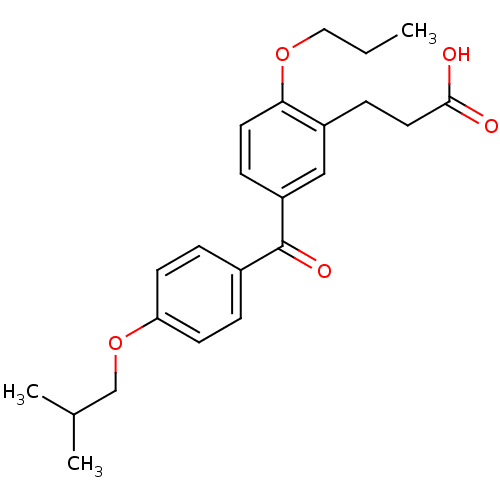 (3-[5-(4-isobutoxybenzoyl)-2-propoxyphenyl]propioni...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180046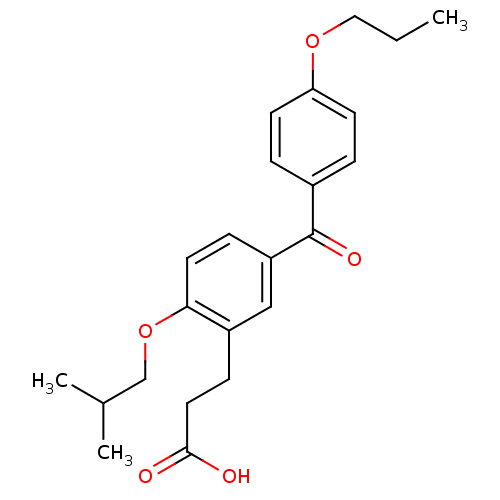 (3-[2-isobutoxy-5-(4-propoxybenzoyl)phenyl]propioni...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180053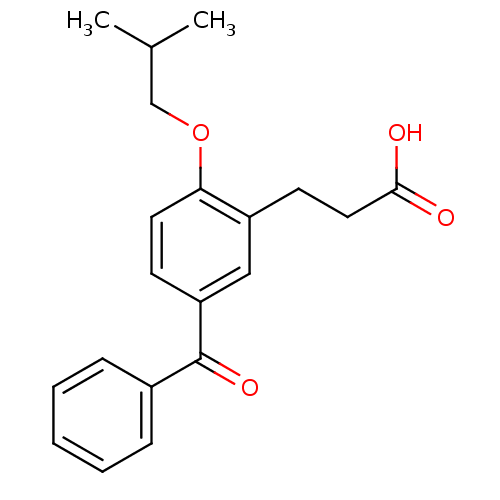 (3-(5-benzoyl-2-isobutoxyphenyl)propionic acid | CH...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein c-Fos/Transcription factor Jun (Homo sapiens (Human)) | BDBM50180045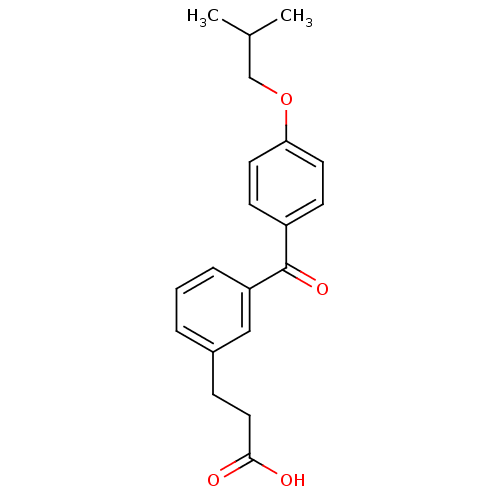 (3-[3-(4-isobutoxybenzoyl)phenyl]propionic acid | C...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Chemical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity on AP1 using ELISA based AP1 DNA binding | J Med Chem 49: 80-91 (2006) Article DOI: 10.1021/jm050550d BindingDB Entry DOI: 10.7270/Q2VX0G2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||