

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11694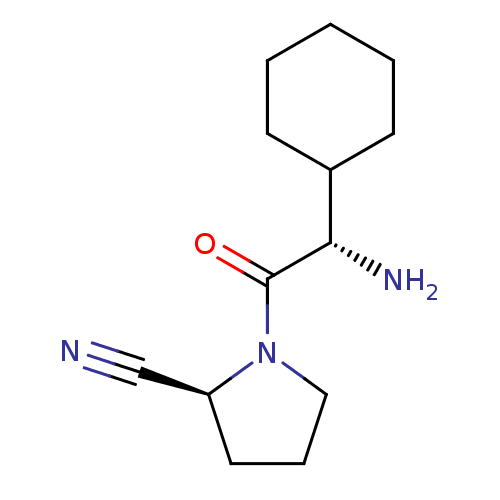 ((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11719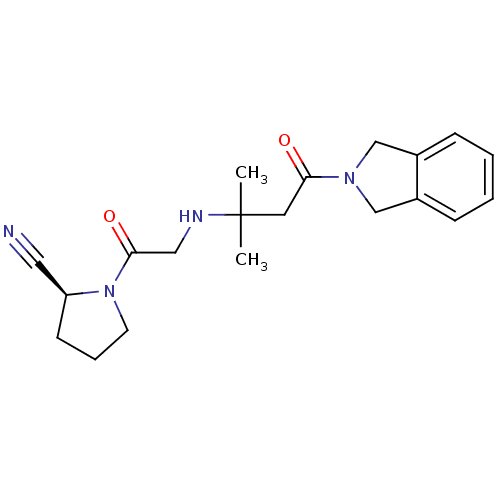 ((2S)-1-(2-{[4-(2,3-dihydro-1H-isoindol-2-yl)-2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11718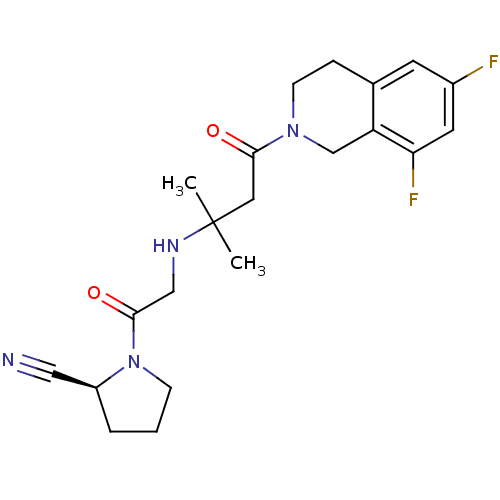 ((2S)-1-(2-{[4-(6,8-difluoro-1,2,3,4-tetrahydroisoq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11694 ((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11717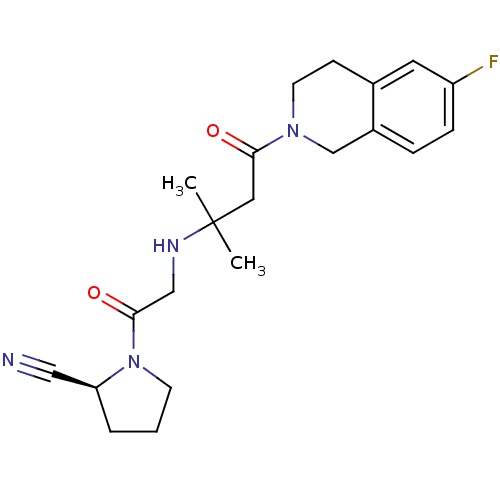 ((2S)-1-(2-{[4-(6-fluoro-1,2,3,4-tetrahydroisoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11716 ((2S)-1-(2-{[2-methyl-4-oxo-4-(1,2,3,4-tetrahydrois...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11695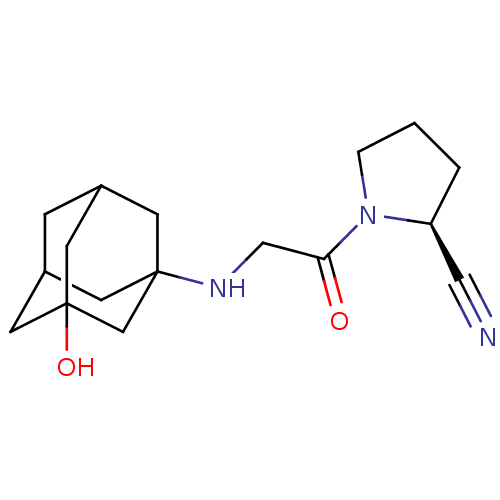 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11113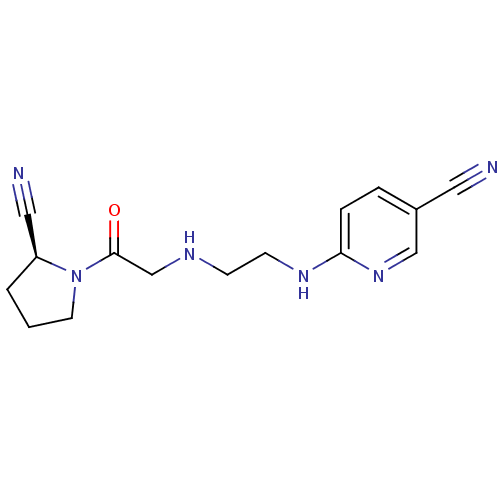 (6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11714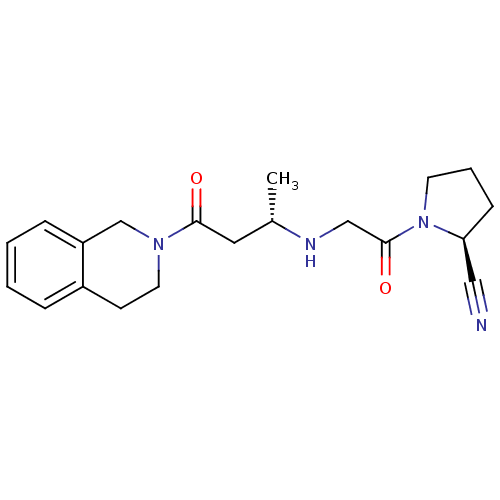 ((2S)-1-(2-{[(2S)-4-oxo-4-(1,2,3,4-tetrahydroisoqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11699 ((2S)-1-(2-{[3-(6-fluoro-1,2,3,4-tetrahydroisoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11697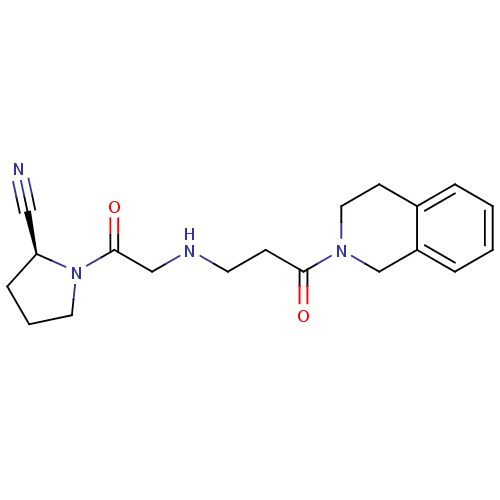 ((2S)-1-(2-{[3-oxo-3-(1,2,3,4-tetrahydroisoquinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11710 ((2S)-Cyanopyrrolidine analogue 18n | 3-({2-[(2S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11700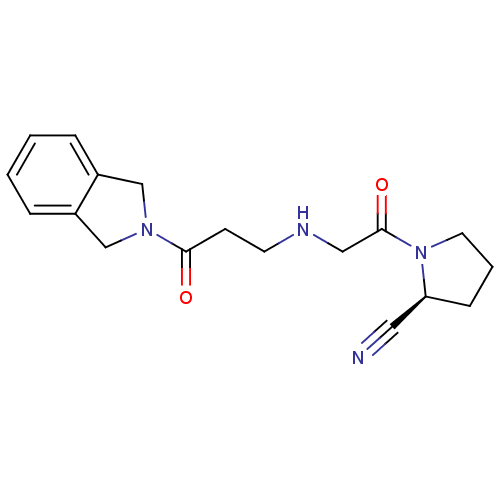 ((2S)-1-(2-{[3-(2,3-dihydro-1H-isoindol-2-yl)-3-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11701 ((2S)-1-{2-[(3-{4-[(3,5-difluorophenyl)carbonyl]pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11712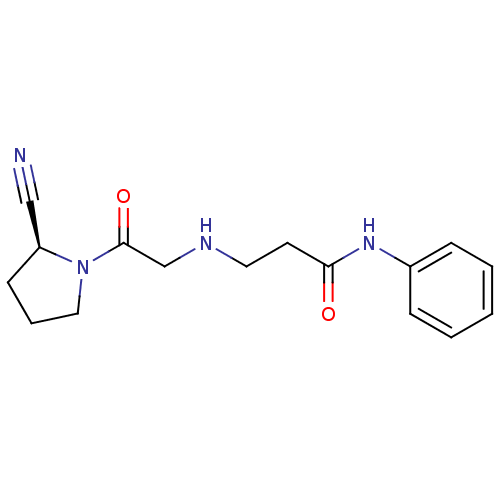 ((2S)-Cyanopyrrolidine analogue 18p | 3-({2-[(2S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11706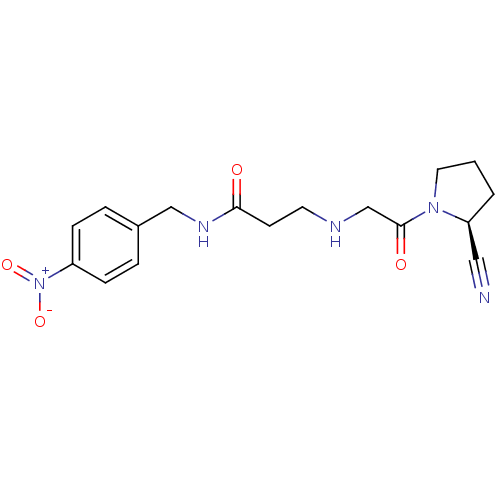 ((2S)-Cyanopyrrolidine analogue 18j | 3-({2-[(2S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 317 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11708 ((2S)-Cyanopyrrolidine analogue 18l | 3-({2-[(2S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11713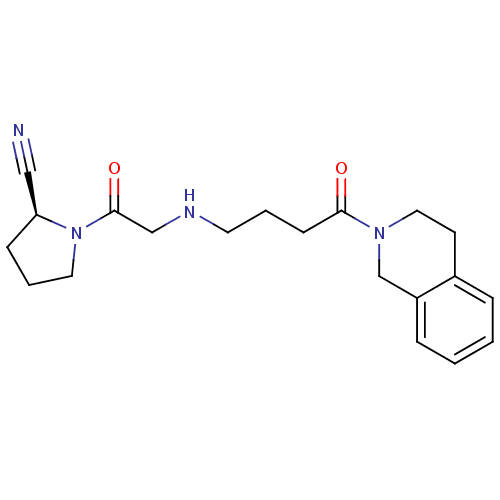 ((2S)-1-(2-{[4-oxo-4-(1,2,3,4-tetrahydroisoquinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 428 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11707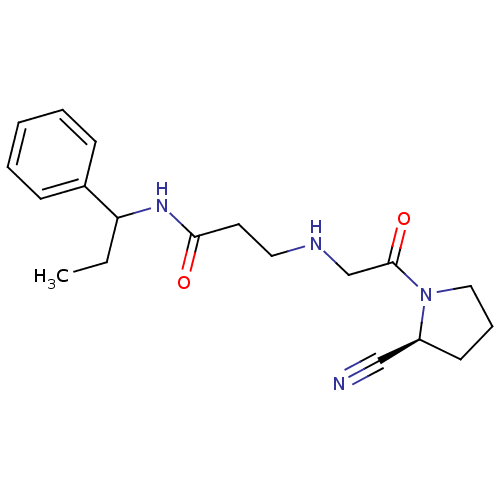 ((2S)-Cyanopyrrolidine analogue 18k | 3-({2-[(2S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 447 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11705 ((2S)-Cyanopyrrolidine analogue 18i | CHEMBL382907 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 452 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11704 ((2S)-1-[2-({3-[4-(1,3-benzothiazol-2-yl)piperazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 527 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11711 ((2S)-Cyanopyrrolidine analogue 18o | 3-({2-[(2S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 564 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11703 ((2S)-Cyanopyrrolidine analogue 18g | 1-{4-[3-({2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 629 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11698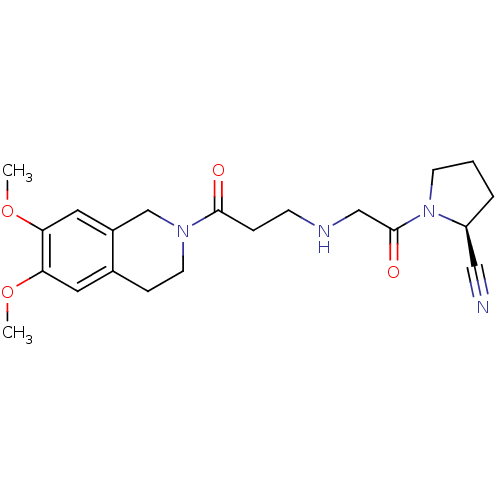 ((2S)-1-(2-{[3-(6,7-dimethoxy-1,2,3,4-tetrahydroiso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 651 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11701 ((2S)-1-{2-[(3-{4-[(3,5-difluorophenyl)carbonyl]pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 676 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11709 ((2S)-Cyanopyrrolidine analogue 18m | 3-({2-[(2S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 784 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11715 ((2S)-1-(2-{[(3R)-4-methyl-1-oxo-1-(1,2,3,4-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 811 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11712 ((2S)-Cyanopyrrolidine analogue 18p | 3-({2-[(2S)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 855 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11720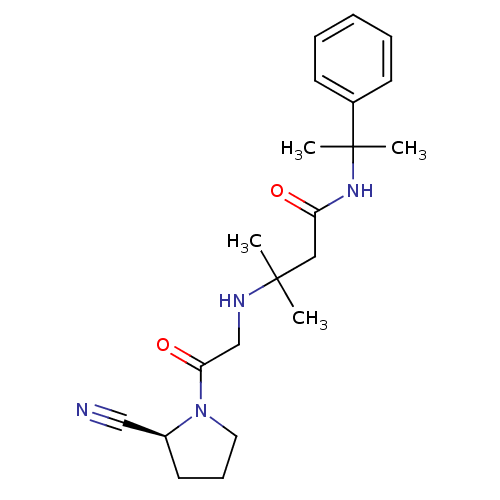 ((2S)-Cyanopyrrolidine analogue 21e | 3-({2-[(2S)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11713 ((2S)-1-(2-{[4-oxo-4-(1,2,3,4-tetrahydroisoquinolin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11702 ((2S)-Cyanopyrrolidine analogue 18f | 1-[3-[2-[2-Cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11464 ((2S,3S)-2-amino-3-methyl-1-(1,3-thiazolidin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11699 ((2S)-1-(2-{[3-(6-fluoro-1,2,3,4-tetrahydroisoquino...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11703 ((2S)-Cyanopyrrolidine analogue 18g | 1-{4-[3-({2-[...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11704 ((2S)-1-[2-({3-[4-(1,3-benzothiazol-2-yl)piperazin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11700 ((2S)-1-(2-{[3-(2,3-dihydro-1H-isoindol-2-yl)-3-oxo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11464 ((2S,3S)-2-amino-3-methyl-1-(1,3-thiazolidin-3-yl)p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11706 ((2S)-Cyanopyrrolidine analogue 18j | 3-({2-[(2S)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11711 ((2S)-Cyanopyrrolidine analogue 18o | 3-({2-[(2S)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11696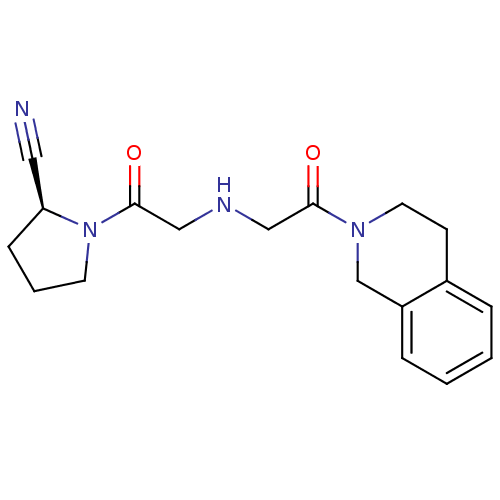 ((2S)-1-(2-{[2-oxo-2-(1,2,3,4-tetrahydroisoquinolin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11698 ((2S)-1-(2-{[3-(6,7-dimethoxy-1,2,3,4-tetrahydroiso...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11702 ((2S)-Cyanopyrrolidine analogue 18f | 1-[3-[2-[2-Cy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11697 ((2S)-1-(2-{[3-oxo-3-(1,2,3,4-tetrahydroisoquinolin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11696 ((2S)-1-(2-{[2-oxo-2-(1,2,3,4-tetrahydroisoquinolin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11113 (6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11714 ((2S)-1-(2-{[(2S)-4-oxo-4-(1,2,3,4-tetrahydroisoqui...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11708 ((2S)-Cyanopyrrolidine analogue 18l | 3-({2-[(2S)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11704 ((2S)-1-[2-({3-[4-(1,3-benzothiazol-2-yl)piperazin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11710 ((2S)-Cyanopyrrolidine analogue 18n | 3-({2-[(2S)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11702 ((2S)-Cyanopyrrolidine analogue 18f | 1-[3-[2-[2-Cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11705 ((2S)-Cyanopyrrolidine analogue 18i | CHEMBL382907 ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11709 ((2S)-Cyanopyrrolidine analogue 18m | 3-({2-[(2S)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11705 ((2S)-Cyanopyrrolidine analogue 18i | CHEMBL382907 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11703 ((2S)-Cyanopyrrolidine analogue 18g | 1-{4-[3-({2-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11714 ((2S)-1-(2-{[(2S)-4-oxo-4-(1,2,3,4-tetrahydroisoqui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11711 ((2S)-Cyanopyrrolidine analogue 18o | 3-({2-[(2S)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11707 ((2S)-Cyanopyrrolidine analogue 18k | 3-({2-[(2S)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11113 (6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11709 ((2S)-Cyanopyrrolidine analogue 18m | 3-({2-[(2S)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11718 ((2S)-1-(2-{[4-(6,8-difluoro-1,2,3,4-tetrahydroisoq...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11713 ((2S)-1-(2-{[4-oxo-4-(1,2,3,4-tetrahydroisoquinolin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11716 ((2S)-1-(2-{[2-methyl-4-oxo-4-(1,2,3,4-tetrahydrois...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11708 ((2S)-Cyanopyrrolidine analogue 18l | 3-({2-[(2S)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11706 ((2S)-Cyanopyrrolidine analogue 18j | 3-({2-[(2S)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11694 ((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11715 ((2S)-1-(2-{[(3R)-4-methyl-1-oxo-1-(1,2,3,4-tetrahy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11717 ((2S)-1-(2-{[4-(6-fluoro-1,2,3,4-tetrahydroisoquino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11712 ((2S)-Cyanopyrrolidine analogue 18p | 3-({2-[(2S)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11464 ((2S,3S)-2-amino-3-methyl-1-(1,3-thiazolidin-3-yl)p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11701 ((2S)-1-{2-[(3-{4-[(3,5-difluorophenyl)carbonyl]pip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11720 ((2S)-Cyanopyrrolidine analogue 21e | 3-({2-[(2S)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11696 ((2S)-1-(2-{[2-oxo-2-(1,2,3,4-tetrahydroisoquinolin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11698 ((2S)-1-(2-{[3-(6,7-dimethoxy-1,2,3,4-tetrahydroiso...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11715 ((2S)-1-(2-{[(3R)-4-methyl-1-oxo-1-(1,2,3,4-tetrahy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11719 ((2S)-1-(2-{[4-(2,3-dihydro-1H-isoindol-2-yl)-2-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11720 ((2S)-Cyanopyrrolidine analogue 21e | 3-({2-[(2S)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11716 ((2S)-1-(2-{[2-methyl-4-oxo-4-(1,2,3,4-tetrahydrois...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11718 ((2S)-1-(2-{[4-(6,8-difluoro-1,2,3,4-tetrahydroisoq...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11699 ((2S)-1-(2-{[3-(6-fluoro-1,2,3,4-tetrahydroisoquino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11700 ((2S)-1-(2-{[3-(2,3-dihydro-1H-isoindol-2-yl)-3-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11697 ((2S)-1-(2-{[3-oxo-3-(1,2,3,4-tetrahydroisoquinolin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11695 ((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11719 ((2S)-1-(2-{[4-(2,3-dihydro-1H-isoindol-2-yl)-2-met...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11707 ((2S)-Cyanopyrrolidine analogue 18k | 3-({2-[(2S)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM11717 ((2S)-1-(2-{[4-(6-fluoro-1,2,3,4-tetrahydroisoquino...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM11710 ((2S)-Cyanopyrrolidine analogue 18n | 3-({2-[(2S)-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes | Assay Description The enzyme activity resulted in the liberation of free pNA at 405 nm. Reaction progress was monitored using a Molecular Devices SpectraMax Plus micro... | J Med Chem 49: 373-80 (2006) Article DOI: 10.1021/jm0507781 BindingDB Entry DOI: 10.7270/Q28W3BJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||