 Found 68 hits of Enzyme Inhibition Constant Data
Found 68 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180334

(2-(3,4-Methylenedioxyphenylamino)-6-(3-acetamidoph...)Show InChI InChI=1S/C19H16N4O3/c1-12(24)21-14-4-2-3-13(7-14)16-9-20-10-19(23-16)22-15-5-6-17-18(8-15)26-11-25-17/h2-10H,11H2,1H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180359

(2-(3-Oxo-1,3-dihydroisobenzofuran-5-ylamino)-6-(3-...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc3COC(=O)c3c2)n1 Show InChI InChI=1S/C20H16N4O3/c1-12(25)22-15-4-2-3-13(7-15)18-9-21-10-19(24-18)23-16-6-5-14-11-27-20(26)17(14)8-16/h2-10H,11H2,1H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180359

(2-(3-Oxo-1,3-dihydroisobenzofuran-5-ylamino)-6-(3-...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc3COC(=O)c3c2)n1 Show InChI InChI=1S/C20H16N4O3/c1-12(25)22-15-4-2-3-13(7-15)18-9-21-10-19(24-18)23-16-6-5-14-11-27-20(26)17(14)8-16/h2-10H,11H2,1H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180350
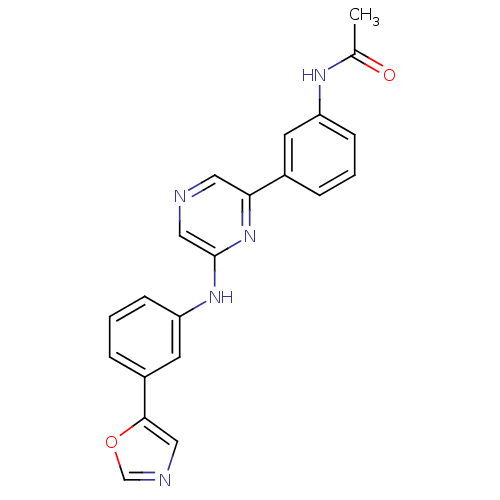
(2-[3-(Oxazol-5-yl)phenylamino]-6-(3-acetamidopheny...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2cccc(c2)-c2cnco2)n1 Show InChI InChI=1S/C21H17N5O2/c1-14(27)24-17-6-2-4-15(8-17)19-10-22-12-21(26-19)25-18-7-3-5-16(9-18)20-11-23-13-28-20/h2-13H,1H3,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180353
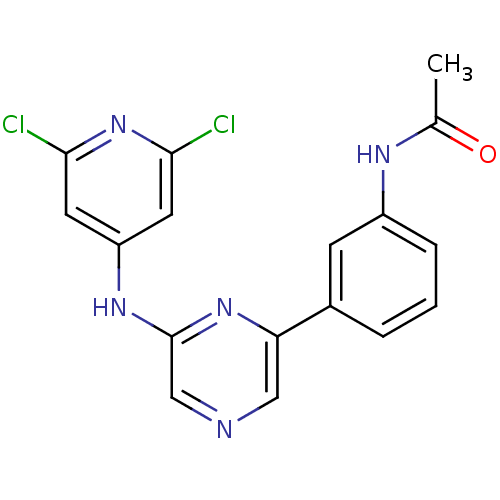
(2-(2,6-Dichloropyridin-4-ylamino)-6-(3-acetamidoph...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2cc(Cl)nc(Cl)c2)n1 Show InChI InChI=1S/C17H13Cl2N5O/c1-10(25)21-12-4-2-3-11(5-12)14-8-20-9-17(23-14)22-13-6-15(18)24-16(19)7-13/h2-9H,1H3,(H,21,25)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180339

(2-(3,4-Dimethoxyphenylamino)-6-(3-acetamidophenyl)...)Show SMILES COc1ccc(Nc2cncc(n2)-c2cccc(NC(C)=O)c2)cc1OC Show InChI InChI=1S/C20H20N4O3/c1-13(25)22-15-6-4-5-14(9-15)17-11-21-12-20(24-17)23-16-7-8-18(26-2)19(10-16)27-3/h4-12H,1-3H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180330
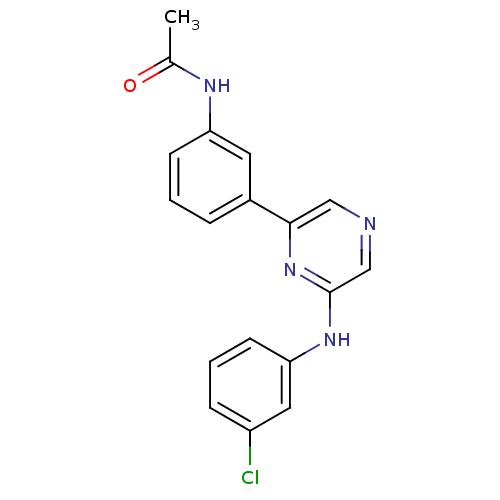
(2-(3-Chlorophenylamino)-6-(3-acetamidophenyl)pyraz...)Show InChI InChI=1S/C18H15ClN4O/c1-12(24)21-15-6-2-4-13(8-15)17-10-20-11-18(23-17)22-16-7-3-5-14(19)9-16/h2-11H,1H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180354
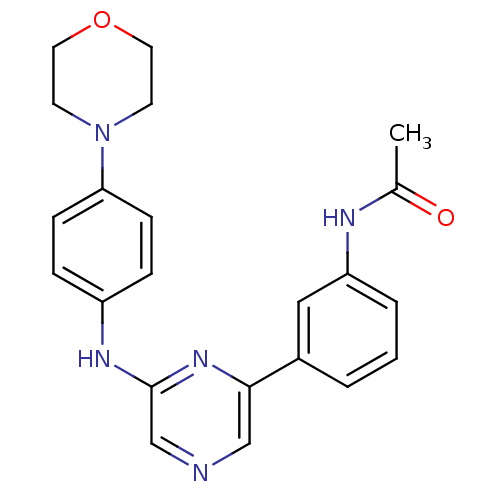
(2-(4-Morpholinophenylamino)-6-(3-acetamidophenyl)p...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O2/c1-16(28)24-19-4-2-3-17(13-19)21-14-23-15-22(26-21)25-18-5-7-20(8-6-18)27-9-11-29-12-10-27/h2-8,13-15H,9-12H2,1H3,(H,24,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180331

(2-(3,5-Dimethoxyphenylamino)-6-(3-acetamidophenyl)...)Show SMILES COc1cc(Nc2cncc(n2)-c2cccc(NC(C)=O)c2)cc(OC)c1 Show InChI InChI=1S/C20H20N4O3/c1-13(25)22-15-6-4-5-14(7-15)19-11-21-12-20(24-19)23-16-8-17(26-2)10-18(9-16)27-3/h4-12H,1-3H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180331

(2-(3,5-Dimethoxyphenylamino)-6-(3-acetamidophenyl)...)Show SMILES COc1cc(Nc2cncc(n2)-c2cccc(NC(C)=O)c2)cc(OC)c1 Show InChI InChI=1S/C20H20N4O3/c1-13(25)22-15-6-4-5-14(7-15)19-11-21-12-20(24-19)23-16-8-17(26-2)10-18(9-16)27-3/h4-12H,1-3H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180337
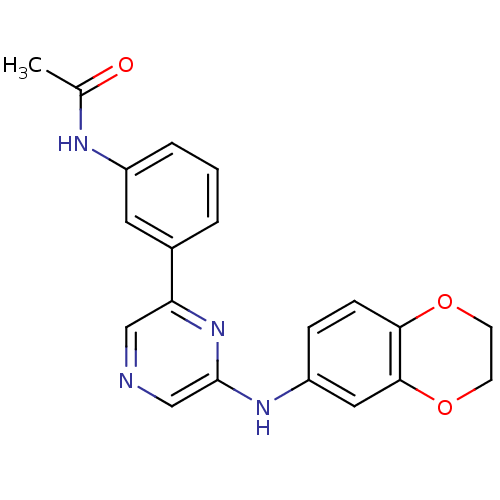
(2-(2,3-Dihydrobenzo[b][1,4]dioxin-6-ylamino)-6-(3-...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc3OCCOc3c2)n1 Show InChI InChI=1S/C20H18N4O3/c1-13(25)22-15-4-2-3-14(9-15)17-11-21-12-20(24-17)23-16-5-6-18-19(10-16)27-8-7-26-18/h2-6,9-12H,7-8H2,1H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180356
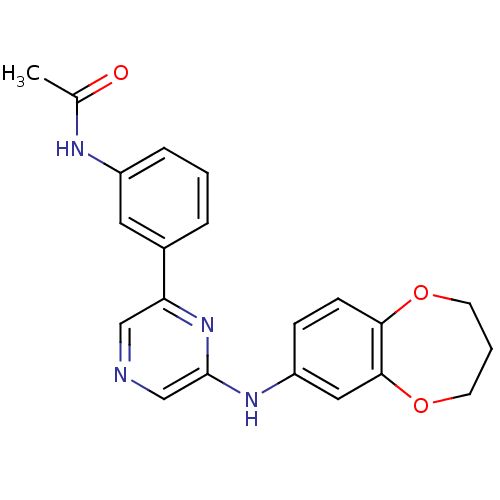
(2-(3,4-Dihydro-2H-benzo[b][1,4]dioxepin-7-ylamino)...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc3OCCCOc3c2)n1 Show InChI InChI=1S/C21H20N4O3/c1-14(26)23-16-5-2-4-15(10-16)18-12-22-13-21(25-18)24-17-6-7-19-20(11-17)28-9-3-8-27-19/h2,4-7,10-13H,3,8-9H2,1H3,(H,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25561

(2,6-Disubstituted Pyrazine, 1 | CHEMBL200114 | N-(...)Show SMILES COc1cc(Nc2cncc(n2)-c2cccc(NC(C)=O)c2)cc(OC)c1OC Show InChI InChI=1S/C21H22N4O4/c1-13(26)23-15-7-5-6-14(8-15)17-11-22-12-20(25-17)24-16-9-18(27-2)21(29-4)19(10-16)28-3/h5-12H,1-4H3,(H,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM25561

(2,6-Disubstituted Pyrazine, 1 | CHEMBL200114 | N-(...)Show SMILES COc1cc(Nc2cncc(n2)-c2cccc(NC(C)=O)c2)cc(OC)c1OC Show InChI InChI=1S/C21H22N4O4/c1-13(26)23-15-7-5-6-14(8-15)17-11-22-12-20(25-17)24-16-9-18(27-2)21(29-4)19(10-16)28-3/h5-12H,1-4H3,(H,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180332
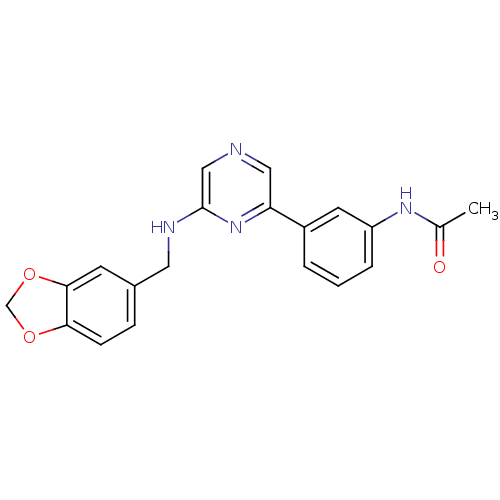
(2-(3,4-Methylenedioxyphenylaminomethyl)-6-(3-aceta...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(NCc2ccc3OCOc3c2)n1 Show InChI InChI=1S/C20H18N4O3/c1-13(25)23-16-4-2-3-15(8-16)17-10-21-11-20(24-17)22-9-14-5-6-18-19(7-14)27-12-26-18/h2-8,10-11H,9,12H2,1H3,(H,22,24)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180358
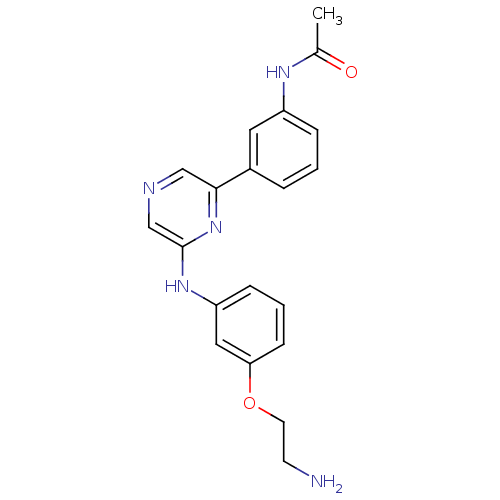
(2-[3-(2-Aminoethoxy)phenylamino]-6-(3-acetamidophe...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2cccc(OCCN)c2)n1 Show InChI InChI=1S/C20H21N5O2/c1-14(26)23-16-5-2-4-15(10-16)19-12-22-13-20(25-19)24-17-6-3-7-18(11-17)27-9-8-21/h2-7,10-13H,8-9,21H2,1H3,(H,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180341
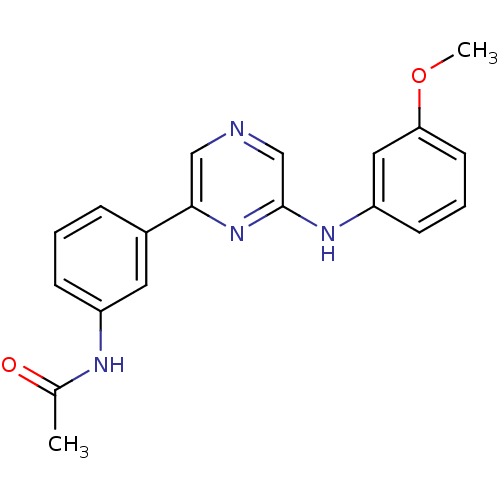
(2-(3-Methoxyphenylamino)-6-(3-acetamidophenyl)pyra...)Show InChI InChI=1S/C19H18N4O2/c1-13(24)21-15-6-3-5-14(9-15)18-11-20-12-19(23-18)22-16-7-4-8-17(10-16)25-2/h3-12H,1-2H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180333
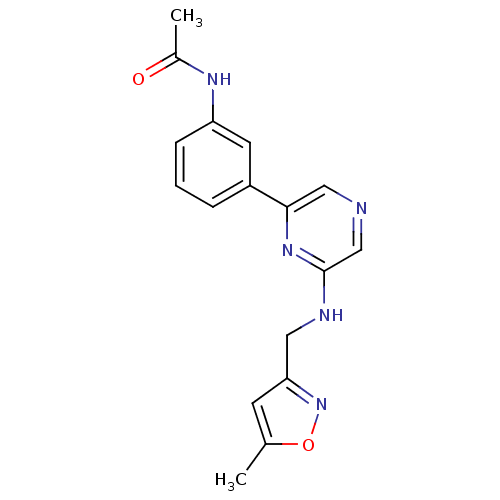
(2-[(5-Methyl-3-isoxazolyl)methylamino]-6-(3-acetam...)Show InChI InChI=1S/C17H17N5O2/c1-11-6-15(22-24-11)8-19-17-10-18-9-16(21-17)13-4-3-5-14(7-13)20-12(2)23/h3-7,9-10H,8H2,1-2H3,(H,19,21)(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180356

(2-(3,4-Dihydro-2H-benzo[b][1,4]dioxepin-7-ylamino)...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc3OCCCOc3c2)n1 Show InChI InChI=1S/C21H20N4O3/c1-14(26)23-16-5-2-4-15(10-16)18-12-22-13-21(25-18)24-17-6-7-19-20(11-17)28-9-3-8-27-19/h2,4-7,10-13H,3,8-9H2,1H3,(H,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180341

(2-(3-Methoxyphenylamino)-6-(3-acetamidophenyl)pyra...)Show InChI InChI=1S/C19H18N4O2/c1-13(24)21-15-6-3-5-14(9-15)18-11-20-12-19(23-18)22-16-7-4-8-17(10-16)25-2/h3-12H,1-2H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180355

(2-[4-(Trifluoromethoxy)phenylamino]-6-(3-acetamido...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc(OC(F)(F)F)cc2)n1 Show InChI InChI=1S/C19H15F3N4O2/c1-12(27)24-15-4-2-3-13(9-15)17-10-23-11-18(26-17)25-14-5-7-16(8-6-14)28-19(20,21)22/h2-11H,1H3,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180358

(2-[3-(2-Aminoethoxy)phenylamino]-6-(3-acetamidophe...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2cccc(OCCN)c2)n1 Show InChI InChI=1S/C20H21N5O2/c1-14(26)23-16-5-2-4-15(10-16)19-12-22-13-20(25-19)24-17-6-3-7-18(11-17)27-9-8-21/h2-7,10-13H,8-9,21H2,1H3,(H,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180337

(2-(2,3-Dihydrobenzo[b][1,4]dioxin-6-ylamino)-6-(3-...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc3OCCOc3c2)n1 Show InChI InChI=1S/C20H18N4O3/c1-13(25)22-15-4-2-3-14(9-15)17-11-21-12-20(24-17)23-16-5-6-18-19(10-16)27-8-7-26-18/h2-6,9-12H,7-8H2,1H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180339

(2-(3,4-Dimethoxyphenylamino)-6-(3-acetamidophenyl)...)Show SMILES COc1ccc(Nc2cncc(n2)-c2cccc(NC(C)=O)c2)cc1OC Show InChI InChI=1S/C20H20N4O3/c1-13(25)22-15-6-4-5-14(9-15)17-11-21-12-20(24-17)23-16-7-8-18(26-2)19(10-16)27-3/h4-12H,1-3H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180352

(2-(Phenylamino)-6-(3-acetamidophenyl)pyrazine | CH...)Show InChI InChI=1S/C18H16N4O/c1-13(23)20-16-9-5-6-14(10-16)17-11-19-12-18(22-17)21-15-7-3-2-4-8-15/h2-12H,1H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180344
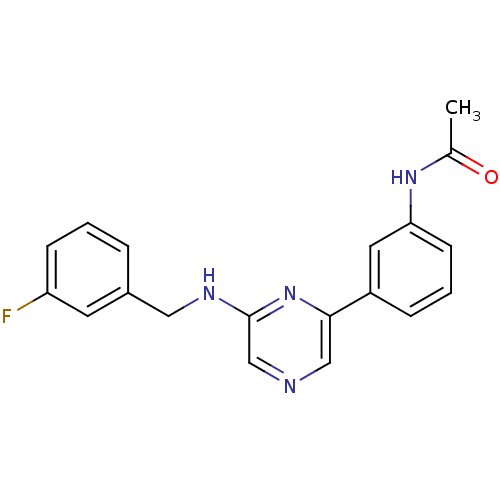
(2-(3-Fluorophenylmethylamino)-6-(3'-acetamidopheny...)Show InChI InChI=1S/C19H17FN4O/c1-13(25)23-17-7-3-5-15(9-17)18-11-21-12-19(24-18)22-10-14-4-2-6-16(20)8-14/h2-9,11-12H,10H2,1H3,(H,22,24)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180357

(2-[4-(Morpholinosulfonyl)phenylamino]-6-(3-acetami...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc(cc2)S(=O)(=O)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O4S/c1-16(28)24-19-4-2-3-17(13-19)21-14-23-15-22(26-21)25-18-5-7-20(8-6-18)32(29,30)27-9-11-31-12-10-27/h2-8,13-15H,9-12H2,1H3,(H,24,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180357

(2-[4-(Morpholinosulfonyl)phenylamino]-6-(3-acetami...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc(cc2)S(=O)(=O)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O4S/c1-16(28)24-19-4-2-3-17(13-19)21-14-23-15-22(26-21)25-18-5-7-20(8-6-18)32(29,30)27-9-11-31-12-10-27/h2-8,13-15H,9-12H2,1H3,(H,24,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180330

(2-(3-Chlorophenylamino)-6-(3-acetamidophenyl)pyraz...)Show InChI InChI=1S/C18H15ClN4O/c1-12(24)21-15-6-2-4-13(8-15)17-10-20-11-18(23-17)22-16-7-3-5-14(19)9-16/h2-11H,1H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180351
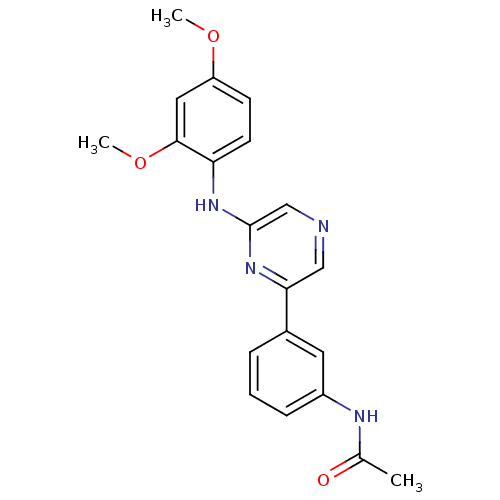
(2-(2,4-Dimethoxyphenylamino)-6-(3-acetamidophenyl)...)Show SMILES COc1ccc(Nc2cncc(n2)-c2cccc(NC(C)=O)c2)c(OC)c1 Show InChI InChI=1S/C20H20N4O3/c1-13(25)22-15-6-4-5-14(9-15)18-11-21-12-20(24-18)23-17-8-7-16(26-2)10-19(17)27-3/h4-12H,1-3H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180334

(2-(3,4-Methylenedioxyphenylamino)-6-(3-acetamidoph...)Show InChI InChI=1S/C19H16N4O3/c1-12(24)21-14-4-2-3-13(7-14)16-9-20-10-19(23-16)22-15-5-6-17-18(8-15)26-11-25-17/h2-10H,11H2,1H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180354

(2-(4-Morpholinophenylamino)-6-(3-acetamidophenyl)p...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc(cc2)N2CCOCC2)n1 Show InChI InChI=1S/C22H23N5O2/c1-16(28)24-19-4-2-3-17(13-19)21-14-23-15-22(26-21)25-18-5-7-20(8-6-18)27-9-11-29-12-10-27/h2-8,13-15H,9-12H2,1H3,(H,24,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180352

(2-(Phenylamino)-6-(3-acetamidophenyl)pyrazine | CH...)Show InChI InChI=1S/C18H16N4O/c1-13(23)20-16-9-5-6-14(10-16)17-11-19-12-18(22-17)21-15-7-3-2-4-8-15/h2-12H,1H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180360
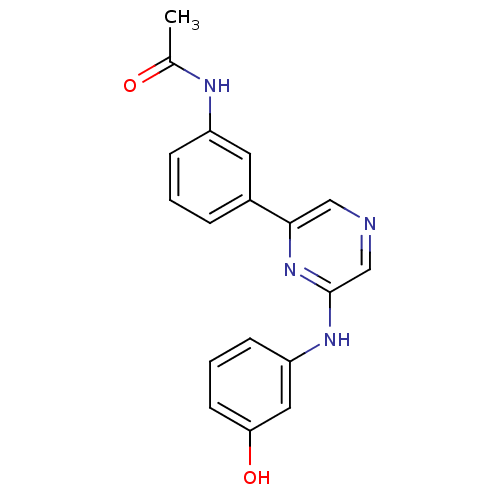
(2-(3-Hydroxyphenylamino)-6-(3-acetamidophenyl)pyra...)Show InChI InChI=1S/C18H16N4O2/c1-12(23)20-14-5-2-4-13(8-14)17-10-19-11-18(22-17)21-15-6-3-7-16(24)9-15/h2-11,24H,1H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180327
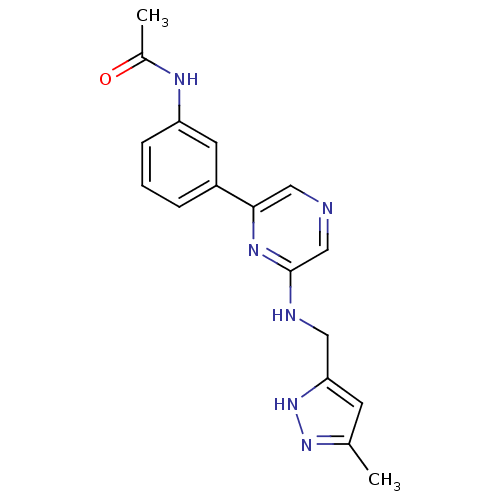
(2-[(1,5-Dimethyl-1H-pyrazol-3-yl)methylamino]-6-(3...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(NCc2cc(C)n[nH]2)n1 Show InChI InChI=1S/C17H18N6O/c1-11-6-15(23-22-11)8-19-17-10-18-9-16(21-17)13-4-3-5-14(7-13)20-12(2)24/h3-7,9-10H,8H2,1-2H3,(H,19,21)(H,20,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180327

(2-[(1,5-Dimethyl-1H-pyrazol-3-yl)methylamino]-6-(3...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(NCc2cc(C)n[nH]2)n1 Show InChI InChI=1S/C17H18N6O/c1-11-6-15(23-22-11)8-19-17-10-18-9-16(21-17)13-4-3-5-14(7-13)20-12(2)24/h3-7,9-10H,8H2,1-2H3,(H,19,21)(H,20,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180363

(2-(Benzylamino)-6-(3-acetamidophenyl)pyrazine | CH...)Show InChI InChI=1S/C19H18N4O/c1-14(24)22-17-9-5-8-16(10-17)18-12-20-13-19(23-18)21-11-15-6-3-2-4-7-15/h2-10,12-13H,11H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180329

(2-(4-tert-Butylthiazol-2-ylamino)-6-(3-acetamidoph...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2nc(cs2)C(C)(C)C)n1 Show InChI InChI=1S/C19H21N5OS/c1-12(25)21-14-7-5-6-13(8-14)15-9-20-10-17(22-15)24-18-23-16(11-26-18)19(2,3)4/h5-11H,1-4H3,(H,21,25)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180351

(2-(2,4-Dimethoxyphenylamino)-6-(3-acetamidophenyl)...)Show SMILES COc1ccc(Nc2cncc(n2)-c2cccc(NC(C)=O)c2)c(OC)c1 Show InChI InChI=1S/C20H20N4O3/c1-13(25)22-15-6-4-5-14(9-15)18-11-21-12-20(24-18)23-17-8-7-16(26-2)10-19(17)27-3/h4-12H,1-3H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180362
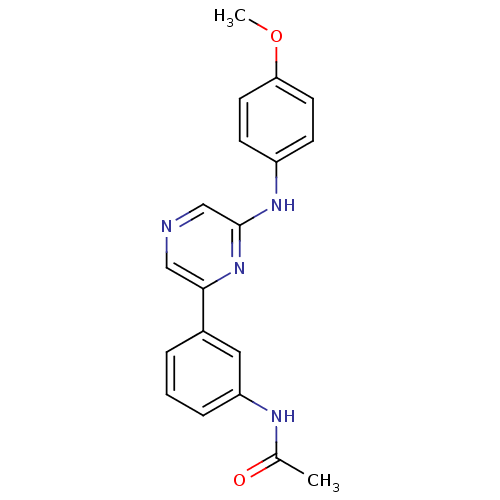
(CHEMBL199798 | N-(3-(6-(4-methoxyphenylamino)pyraz...)Show InChI InChI=1S/C19H18N4O2/c1-13(24)21-16-5-3-4-14(10-16)18-11-20-12-19(23-18)22-15-6-8-17(25-2)9-7-15/h3-12H,1-2H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180347

(2-(3-Chloro-4-fluorobenzylamino)-6-(3-acetamidophe...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(NCc2ccc(F)c(Cl)c2)n1 Show InChI InChI=1S/C19H16ClFN4O/c1-12(26)24-15-4-2-3-14(8-15)18-10-22-11-19(25-18)23-9-13-5-6-17(21)16(20)7-13/h2-8,10-11H,9H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180338

(2-(3-Phenylpropylamino)-6-(3-acetamidophenyl)pyraz...)Show InChI InChI=1S/C21H22N4O/c1-16(26)24-19-11-5-10-18(13-19)20-14-22-15-21(25-20)23-12-6-9-17-7-3-2-4-8-17/h2-5,7-8,10-11,13-15H,6,9,12H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180342
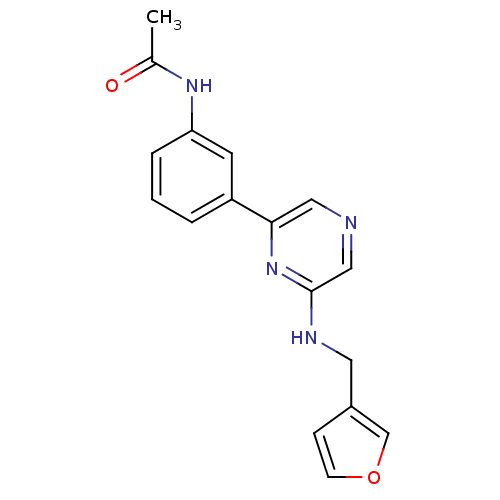
(2-(3-Furylmethylamino)-6-(3-acetamidophenyl)pyrazi...)Show InChI InChI=1S/C17H16N4O2/c1-12(22)20-15-4-2-3-14(7-15)16-9-18-10-17(21-16)19-8-13-5-6-23-11-13/h2-7,9-11H,8H2,1H3,(H,19,21)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180340
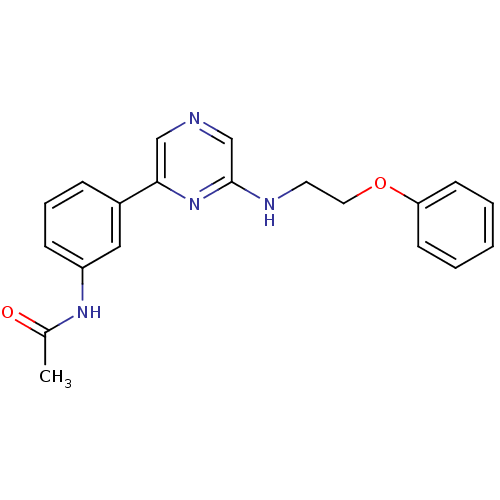
(2-Phenoxyethylamino-6-(3-acetamidophenyl)pyrazine ...)Show InChI InChI=1S/C20H20N4O2/c1-15(25)23-17-7-5-6-16(12-17)19-13-21-14-20(24-19)22-10-11-26-18-8-3-2-4-9-18/h2-9,12-14H,10-11H2,1H3,(H,22,24)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180365
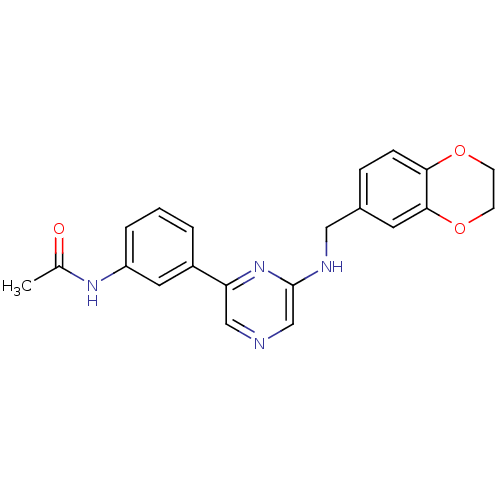
(2-(2,3-Dehydro-1,4-benzodioxin-6-methylamino)-6-(3...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(NCc2ccc3OCCOc3c2)n1 Show InChI InChI=1S/C21H20N4O3/c1-14(26)24-17-4-2-3-16(10-17)18-12-22-13-21(25-18)23-11-15-5-6-19-20(9-15)28-8-7-27-19/h2-6,9-10,12-13H,7-8,11H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180345

(2-(2,2-Difluorobenzo[d][1,3]dioxol-5-ylamino)-6-(3...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc3OC(F)(F)Oc3c2)n1 Show InChI InChI=1S/C19H14F2N4O3/c1-11(26)23-13-4-2-3-12(7-13)15-9-22-10-18(25-15)24-14-5-6-16-17(8-14)28-19(20,21)27-16/h2-10H,1H3,(H,23,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180336
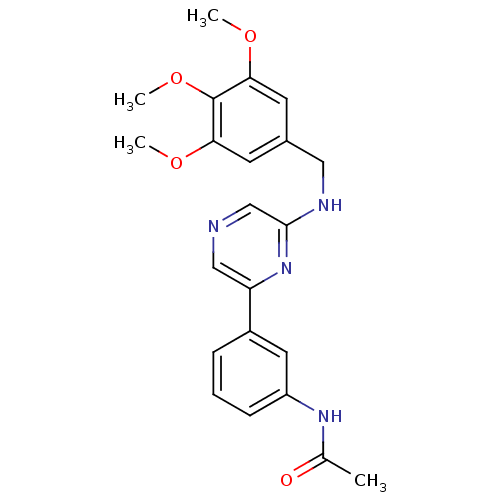
(2-(3,4,5-Trimethoxybenzylamino)-6-(3-acetamidophen...)Show SMILES COc1cc(CNc2cncc(n2)-c2cccc(NC(C)=O)c2)cc(OC)c1OC Show InChI InChI=1S/C22H24N4O4/c1-14(27)25-17-7-5-6-16(10-17)18-12-23-13-21(26-18)24-11-15-8-19(28-2)22(30-4)20(9-15)29-3/h5-10,12-13H,11H2,1-4H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180333

(2-[(5-Methyl-3-isoxazolyl)methylamino]-6-(3-acetam...)Show InChI InChI=1S/C17H17N5O2/c1-11-6-15(22-24-11)8-19-17-10-18-9-16(21-17)13-4-3-5-14(7-13)20-12(2)23/h3-7,9-10H,8H2,1-2H3,(H,19,21)(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180349
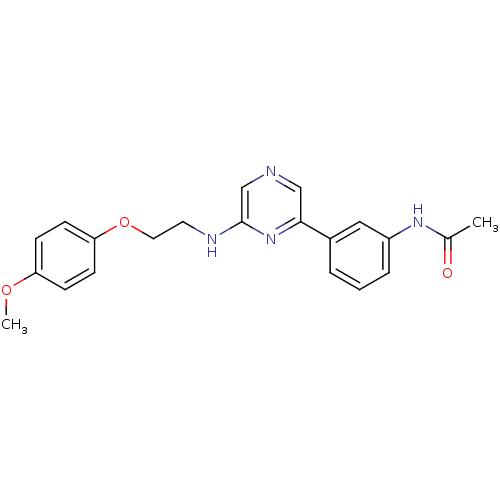
(2-(4-Methoxyphenyloxyethylamino)-6-(3-acetamidophe...)Show SMILES COc1ccc(OCCNc2cncc(n2)-c2cccc(NC(C)=O)c2)cc1 Show InChI InChI=1S/C21H22N4O3/c1-15(26)24-17-5-3-4-16(12-17)20-13-22-14-21(25-20)23-10-11-28-19-8-6-18(27-2)7-9-19/h3-9,12-14H,10-11H2,1-2H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180335

(2-(2,2,3,3-Tetrafluoro-2,3-dihydrobenzo[b][1,4]dio...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc3OC(F)(F)C(F)(F)Oc3c2)n1 Show InChI InChI=1S/C20H14F4N4O3/c1-11(29)26-13-4-2-3-12(7-13)15-9-25-10-18(28-15)27-14-5-6-16-17(8-14)31-20(23,24)19(21,22)30-16/h2-10H,1H3,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180328

(2-(Phenylethylamino)-6-(3-acetamidophenyl)pyrazine...)Show InChI InChI=1S/C20H20N4O/c1-15(25)23-18-9-5-8-17(12-18)19-13-21-14-20(24-19)22-11-10-16-6-3-2-4-7-16/h2-9,12-14H,10-11H2,1H3,(H,22,24)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180350

(2-[3-(Oxazol-5-yl)phenylamino]-6-(3-acetamidopheny...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2cccc(c2)-c2cnco2)n1 Show InChI InChI=1S/C21H17N5O2/c1-14(27)24-17-6-2-4-15(8-17)19-10-22-12-21(26-19)25-18-7-3-5-16(9-18)20-11-23-13-28-20/h2-13H,1H3,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180346

(2-(Phenyl-N-methylamino)-6-(3-acetamidophenyl)pyra...)Show InChI InChI=1S/C19H19N5O/c1-14(25)21-16-8-6-7-15(11-16)18-12-20-13-19(22-18)23-24(2)17-9-4-3-5-10-17/h3-13H,1-2H3,(H,21,25)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180349

(2-(4-Methoxyphenyloxyethylamino)-6-(3-acetamidophe...)Show SMILES COc1ccc(OCCNc2cncc(n2)-c2cccc(NC(C)=O)c2)cc1 Show InChI InChI=1S/C21H22N4O3/c1-15(26)24-17-5-3-4-16(12-17)20-13-22-14-21(25-20)23-10-11-28-19-8-6-18(27-2)7-9-19/h3-9,12-14H,10-11H2,1-2H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180340

(2-Phenoxyethylamino-6-(3-acetamidophenyl)pyrazine ...)Show InChI InChI=1S/C20H20N4O2/c1-15(25)23-17-7-5-6-16(12-17)19-13-21-14-20(24-19)22-10-11-26-18-8-3-2-4-9-18/h2-9,12-14H,10-11H2,1H3,(H,22,24)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180361
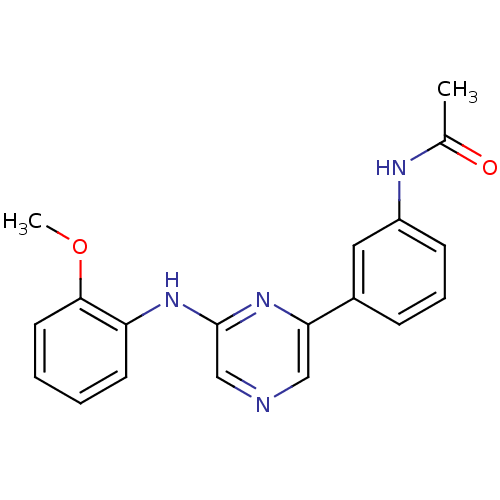
(2-(2-Methoxyphenylamino)-6-(3-acetamidophenyl)pyra...)Show InChI InChI=1S/C19H18N4O2/c1-13(24)21-15-7-5-6-14(10-15)17-11-20-12-19(23-17)22-16-8-3-4-9-18(16)25-2/h3-12H,1-2H3,(H,21,24)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180355

(2-[4-(Trifluoromethoxy)phenylamino]-6-(3-acetamido...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc(OC(F)(F)F)cc2)n1 Show InChI InChI=1S/C19H15F3N4O2/c1-12(27)24-15-4-2-3-13(9-15)17-10-23-11-18(26-17)25-14-5-7-16(8-6-14)28-19(20,21)22/h2-11H,1H3,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180347

(2-(3-Chloro-4-fluorobenzylamino)-6-(3-acetamidophe...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(NCc2ccc(F)c(Cl)c2)n1 Show InChI InChI=1S/C19H16ClFN4O/c1-12(26)24-15-4-2-3-14(8-15)18-10-22-11-19(25-18)23-9-13-5-6-17(21)16(20)7-13/h2-8,10-11H,9H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180336

(2-(3,4,5-Trimethoxybenzylamino)-6-(3-acetamidophen...)Show SMILES COc1cc(CNc2cncc(n2)-c2cccc(NC(C)=O)c2)cc(OC)c1OC Show InChI InChI=1S/C22H24N4O4/c1-14(27)25-17-7-5-6-16(10-17)18-12-23-13-21(26-18)24-11-15-8-19(28-2)22(30-4)20(9-15)29-3/h5-10,12-13H,11H2,1-4H3,(H,24,26)(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180346

(2-(Phenyl-N-methylamino)-6-(3-acetamidophenyl)pyra...)Show InChI InChI=1S/C19H19N5O/c1-14(25)21-16-8-6-7-15(11-16)18-12-20-13-19(22-18)23-24(2)17-9-4-3-5-10-17/h3-13H,1-2H3,(H,21,25)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180348
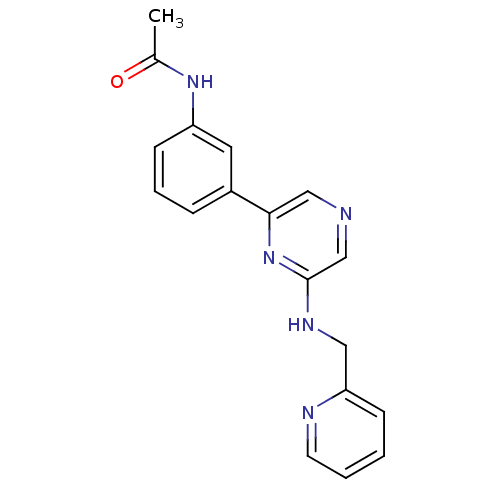
(2-(2-Pyridylmethylamino)-6-(3-acetamidophenyl)pyra...)Show InChI InChI=1S/C18H17N5O/c1-13(24)22-15-7-4-5-14(9-15)17-11-19-12-18(23-17)21-10-16-6-2-3-8-20-16/h2-9,11-12H,10H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180343

(CHEMBL199605 | N-(3-(6-(2,2,3,3-Tetrafluoro-2,3-di...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2cccc3OC(F)(F)C(F)(F)Oc23)n1 Show InChI InChI=1S/C20H14F4N4O3/c1-11(29)26-13-5-2-4-12(8-13)15-9-25-10-17(28-15)27-14-6-3-7-16-18(14)31-20(23,24)19(21,22)30-16/h2-10H,1H3,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Growth inhibition in human melanoma cells WM266.4 expressing mutant B-RAF with SRB |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180338

(2-(3-Phenylpropylamino)-6-(3-acetamidophenyl)pyraz...)Show InChI InChI=1S/C21H22N4O/c1-16(26)24-19-11-5-10-18(13-19)20-14-22-15-21(25-20)23-12-6-9-17-7-3-2-4-8-17/h2-5,7-8,10-11,13-15H,6,9,12H2,1H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180348

(2-(2-Pyridylmethylamino)-6-(3-acetamidophenyl)pyra...)Show InChI InChI=1S/C18H17N5O/c1-13(24)22-15-7-4-5-14(9-15)17-11-19-12-18(23-17)21-10-16-6-2-3-8-20-16/h2-9,11-12H,10H2,1H3,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180364

(2-(3-Chloro-4-fluorophenylamino)-6-(3-acetamidophe...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2ccc(F)c(Cl)c2)n1 Show InChI InChI=1S/C18H14ClFN4O/c1-11(25)22-13-4-2-3-12(7-13)17-9-21-10-18(24-17)23-14-5-6-16(20)15(19)8-14/h2-10H,1H3,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50180343

(CHEMBL199605 | N-(3-(6-(2,2,3,3-Tetrafluoro-2,3-di...)Show SMILES CC(=O)Nc1cccc(c1)-c1cncc(Nc2cccc3OC(F)(F)C(F)(F)Oc23)n1 Show InChI InChI=1S/C20H14F4N4O3/c1-11(29)26-13-5-2-4-12(8-13)15-9-25-10-17(28-15)27-14-6-3-7-16-18(14)31-20(23,24)19(21,22)30-16/h2-10H,1H3,(H,26,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human B-RAF |
J Med Chem 49: 407-16 (2006)
Article DOI: 10.1021/jm050983g
BindingDB Entry DOI: 10.7270/Q2KD1XH9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data