 Found 46 hits of Enzyme Inhibition Constant Data
Found 46 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-C |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-B |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for FLT-3 |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50027186

(CHEMBL377289)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN(CCO)CC#C Show InChI InChI=1S/C28H29FN6O4S/c1-3-8-35(10-11-36)9-5-12-39-25-16-23-22(15-24(25)38-2)27(32-18-31-23)34-28-30-17-21(40-28)14-26(37)33-20-7-4-6-19(29)13-20/h1,4,6-7,13,15-18,36H,5,8-12,14H2,2H3,(H,33,37)(H,30,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50027188
(CHEMBL202241)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C27H29FN6O4S/c1-36-23-14-21-22(15-24(23)38-9-3-6-34-7-10-37-11-8-34)30-17-31-26(21)33-27-29-16-20(39-27)13-25(35)32-19-5-2-4-18(28)12-19/h2,4-5,12,14-17H,3,6-11,13H2,1H3,(H,32,35)(H,29,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
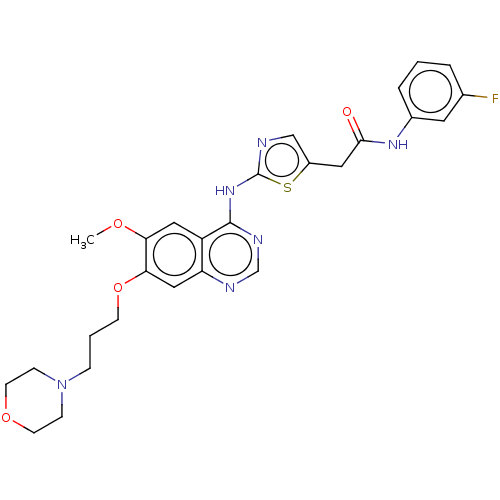
Aurora kinase A
(Homo sapiens (Human)) | BDBM50027190
(CHEMBL202198)Show SMILES CCN(CCO)CCCOc1cc2ncnc(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)c2cc1OC Show InChI InChI=1S/C27H31FN6O4S/c1-3-34(9-10-35)8-5-11-38-24-15-22-21(14-23(24)37-2)26(31-17-30-22)33-27-29-16-20(39-27)13-25(36)32-19-7-4-6-18(28)12-19/h4,6-7,12,14-17,35H,3,5,8-11,13H2,1-2H3,(H,32,36)(H,29,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
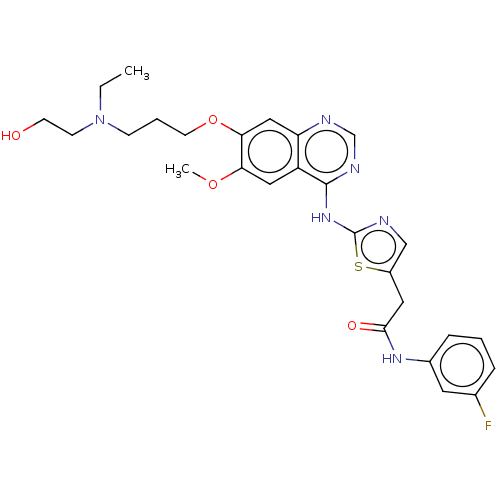
Aurora kinase A
(Homo sapiens (Human)) | BDBM50027228

(CHEMBL201953)Show SMILES COc1cc2c(Nc3ncc(SCc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C28H32FN5O3S2/c1-36-24-13-22-23(14-25(24)37-11-3-8-34-9-6-19(16-35)7-10-34)31-18-32-27(22)33-28-30-15-26(39-28)38-17-20-4-2-5-21(29)12-20/h2,4-5,12-15,18-19,35H,3,6-11,16-17H2,1H3,(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50027191

(CHEMBL202593)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCN1CCC(CCO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-15-23-24(16-26(25)40-12-10-36-8-5-19(6-9-36)7-11-37)32-18-33-28(23)35-29-31-17-22(41-29)14-27(38)34-21-4-2-3-20(30)13-21/h2-4,13,15-19,37H,5-12,14H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181550
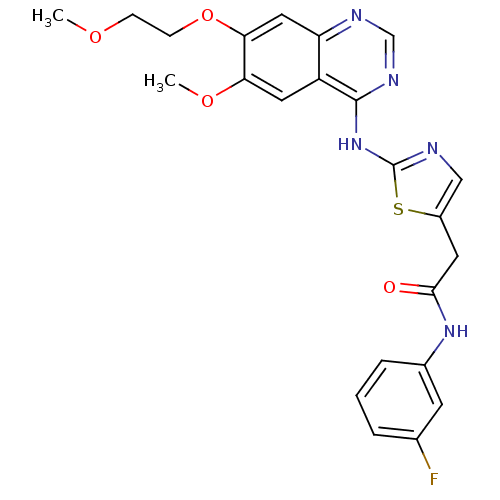
(CHEMBL201297 | N-(3-fluorophenyl)-2-(2-{[6-methoxy...)Show SMILES COCCOc1cc2ncnc(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)c2cc1OC Show InChI InChI=1S/C23H22FN5O4S/c1-31-6-7-33-20-11-18-17(10-19(20)32-2)22(27-13-26-18)29-23-25-12-16(34-23)9-21(30)28-15-5-3-4-14(24)8-15/h3-5,8,10-13H,6-7,9H2,1-2H3,(H,28,30)(H,25,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50027192

(CHEMBL380478)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC[C@H]1CO Show InChI InChI=1S/C28H31FN6O4S/c1-38-24-13-22-23(14-25(24)39-10-4-9-35-8-3-7-20(35)16-36)31-17-32-27(22)34-28-30-15-21(40-28)12-26(37)33-19-6-2-5-18(29)11-19/h2,5-6,11,13-15,17,20,36H,3-4,7-10,12,16H2,1H3,(H,33,37)(H,30,31,32,34)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50027189

(CHEMBL381721)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCN(C)CC1 Show InChI InChI=1S/C28H32FN7O3S/c1-35-8-10-36(11-9-35)7-4-12-39-25-16-23-22(15-24(25)38-2)27(32-18-31-23)34-28-30-17-21(40-28)14-26(37)33-20-6-3-5-19(29)13-20/h3,5-6,13,15-18H,4,7-12,14H2,1-2H3,(H,33,37)(H,30,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181547
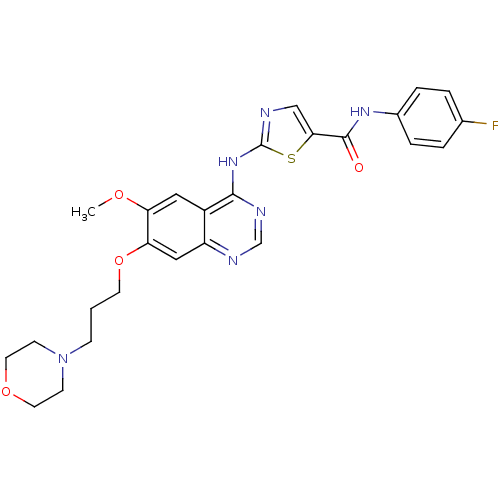
(CHEMBL202567 | N-(4-fluorophenyl)-2-{[6-methoxy-7-...)Show SMILES COc1cc2c(Nc3ncc(s3)C(=O)Nc3ccc(F)cc3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C26H27FN6O4S/c1-35-21-13-19-20(14-22(21)37-10-2-7-33-8-11-36-12-9-33)29-16-30-24(19)32-26-28-15-23(38-26)25(34)31-18-5-3-17(27)4-6-18/h3-6,13-16H,2,7-12H2,1H3,(H,31,34)(H,28,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181532
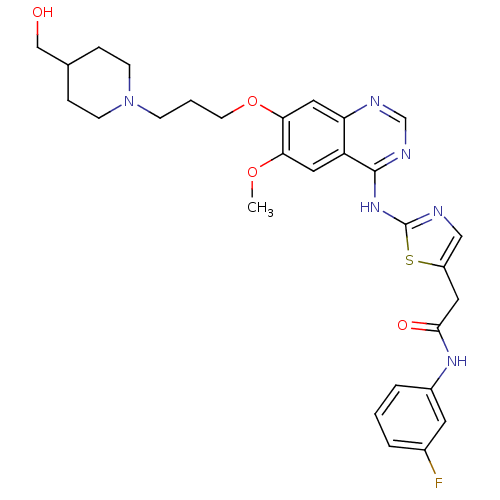
(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181537

(CHEMBL201197 | N-(2,3-difluorophenyl)-2-{2-[(7-{3-...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4F)s3)ncnc2cc1OCCCN1CCC[C@H]1CO Show InChI InChI=1S/C28H30F2N6O4S/c1-39-23-12-19-22(13-24(23)40-10-4-9-36-8-3-5-17(36)15-37)32-16-33-27(19)35-28-31-14-18(41-28)11-25(38)34-21-7-2-6-20(29)26(21)30/h2,6-7,12-14,16-17,37H,3-5,8-11,15H2,1H3,(H,34,38)(H,31,32,33,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181542

(CHEMBL373013 | N-(3-fluorophenyl)-2-(2-(7-(3-(2-hy...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCNCCO Show InChI InChI=1S/C25H27FN6O4S/c1-35-21-12-19-20(13-22(21)36-9-3-6-27-7-8-33)29-15-30-24(19)32-25-28-14-18(37-25)11-23(34)31-17-5-2-4-16(26)10-17/h2,4-5,10,12-15,27,33H,3,6-9,11H2,1H3,(H,31,34)(H,28,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181535

(CHEMBL201655 | N-(5-butyl-1,3-thiazol-2-yl)-6-meth...)Show SMILES CCCCc1cnc(Nc2ncnc3cc(OCCCN4CCOCC4)c(OC)cc23)s1 Show InChI InChI=1S/C23H31N5O3S/c1-3-4-6-17-15-24-23(32-17)27-22-18-13-20(29-2)21(14-19(18)25-16-26-22)31-10-5-7-28-8-11-30-12-9-28/h13-16H,3-12H2,1-2H3,(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181544

(3-fluoro-N-({2-[(7-{3-[4-(hydroxymethyl)piperidin-...)Show SMILES COc1cc2c(Nc3ncc(CNS(=O)(=O)c4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C28H33FN6O5S2/c1-39-25-13-23-24(14-26(25)40-11-3-8-35-9-6-19(17-36)7-10-35)31-18-32-27(23)34-28-30-15-21(41-28)16-33-42(37,38)22-5-2-4-20(29)12-22/h2,4-5,12-15,18-19,33,36H,3,6-11,16-17H2,1H3,(H,30,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181556

(6-methoxy-N-(5-methyl-1,3-thiazol-2-yl)-7-(3-morph...)Show InChI InChI=1S/C20H25N5O3S/c1-14-12-21-20(29-14)24-19-15-10-17(26-2)18(11-16(15)22-13-23-19)28-7-3-4-25-5-8-27-9-6-25/h10-13H,3-9H2,1-2H3,(H,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181540
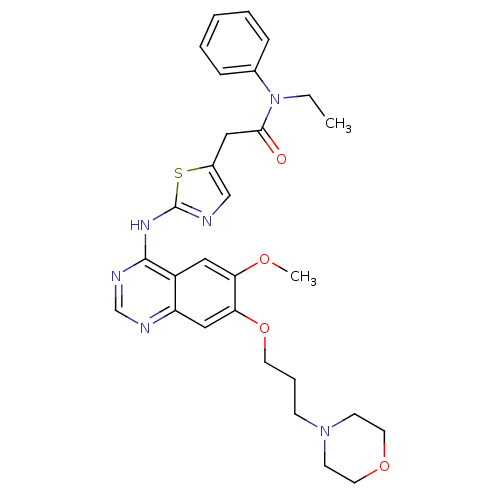
(CHEMBL202026 | N-ethyl-2-(2-{[6-methoxy-7-(3-morph...)Show SMILES CCN(C(=O)Cc1cnc(Nc2ncnc3cc(OCCCN4CCOCC4)c(OC)cc23)s1)c1ccccc1 Show InChI InChI=1S/C29H34N6O4S/c1-3-35(21-8-5-4-6-9-21)27(36)16-22-19-30-29(40-22)33-28-23-17-25(37-2)26(18-24(23)31-20-32-28)39-13-7-10-34-11-14-38-15-12-34/h4-6,8-9,17-20H,3,7,10-16H2,1-2H3,(H,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM14210

(4-(4-(N-benzoylamino)anilino)-6-methoxy-7-(3-(1-mo...)Show SMILES COc1cc2c(Nc3ccc(NC(=O)c4ccccc4)cc3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C29H31N5O4/c1-36-26-18-24-25(19-27(26)38-15-5-12-34-13-16-37-17-14-34)30-20-31-28(24)32-22-8-10-23(11-9-22)33-29(35)21-6-3-2-4-7-21/h2-4,6-11,18-20H,5,12-17H2,1H3,(H,33,35)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of in vitro Aurora-A kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM14210

(4-(4-(N-benzoylamino)anilino)-6-methoxy-7-(3-(1-mo...)Show SMILES COc1cc2c(Nc3ccc(NC(=O)c4ccccc4)cc3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C29H31N5O4/c1-36-26-18-24-25(19-27(26)38-15-5-12-34-13-16-37-17-14-34)30-20-31-28(24)32-22-8-10-23(11-9-22)33-29(35)21-6-3-2-4-7-21/h2-4,6-11,18-20H,5,12-17H2,1H3,(H,33,35)(H,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of in vitro Aurora-B kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181557
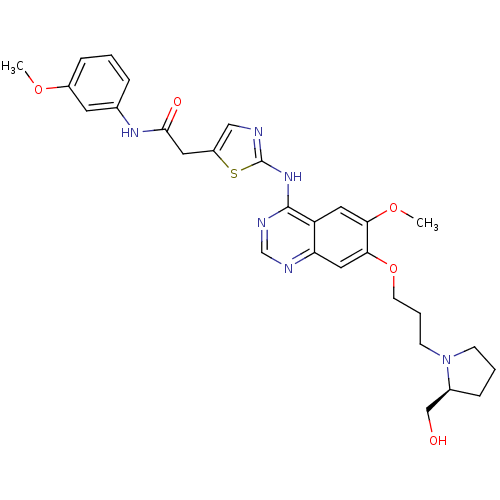
(2-{2-[(7-{3-[(2S)-2-(hydroxymethyl)pyrrolidin-1-yl...)Show SMILES COc1cccc(NC(=O)Cc2cnc(Nc3ncnc4cc(OCCCN5CCC[C@H]5CO)c(OC)cc34)s2)c1 Show InChI InChI=1S/C29H34N6O5S/c1-38-21-8-3-6-19(12-21)33-27(37)13-22-16-30-29(41-22)34-28-23-14-25(39-2)26(15-24(23)31-18-32-28)40-11-5-10-35-9-4-7-20(35)17-36/h3,6,8,12,14-16,18,20,36H,4-5,7,9-11,13,17H2,1-2H3,(H,33,37)(H,30,31,32,34)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181552
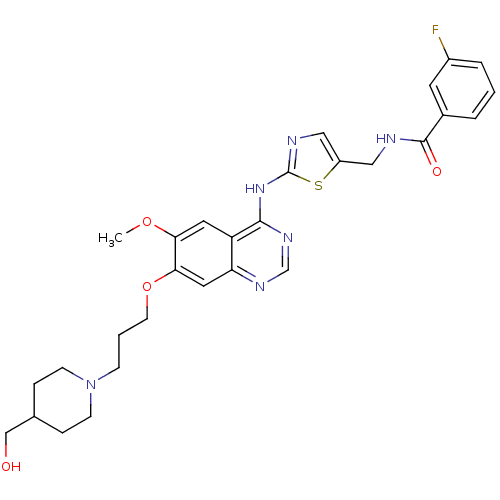
(3-fluoro-N-({2-[(7-{3-[4-(hydroxymethyl)piperidin-...)Show SMILES COc1cc2c(Nc3ncc(CNC(=O)c4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-13-23-24(14-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)33-18-34-27(23)35-29-32-16-22(41-29)15-31-28(38)20-4-2-5-21(30)12-20/h2,4-5,12-14,16,18-19,37H,3,6-11,15,17H2,1H3,(H,31,38)(H,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181554

(2-(2-{[6-methoxy-7-(3-morpholin-4-ylpropoxy)quinaz...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)NCCC(C)C)s3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C26H36N6O4S/c1-18(2)5-6-27-24(33)13-19-16-28-26(37-19)31-25-20-14-22(34-3)23(15-21(20)29-17-30-25)36-10-4-7-32-8-11-35-12-9-32/h14-18H,4-13H2,1-3H3,(H,27,33)(H,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50273571
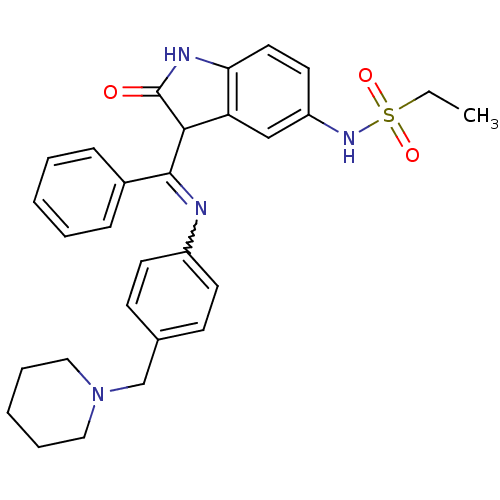
(CHEMBL514409 | Hesperadin | N-(2-oxo-3-(phenyl(4-(...)Show SMILES CCS(=O)(=O)Nc1ccc2NC(=O)C(C(=Nc3ccc(CN4CCCCC4)cc3)c3ccccc3)c2c1 |w:15.15| Show InChI InChI=1S/C29H32N4O3S/c1-2-37(35,36)32-24-15-16-26-25(19-24)27(29(34)31-26)28(22-9-5-3-6-10-22)30-23-13-11-21(12-14-23)20-33-17-7-4-8-18-33/h3,5-6,9-16,19,27,32H,2,4,7-8,17-18,20H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-B immunoprecipitated from mitotic cells |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181538
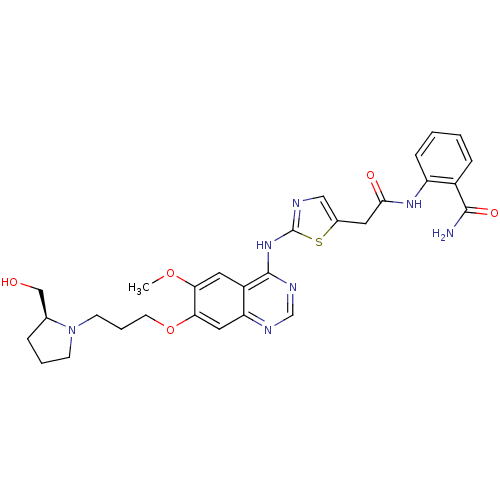
(2-[({2-[(7-{3-[(2S)-2-(hydroxymethyl)pyrrolidin-1-...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4ccccc4C(N)=O)s3)ncnc2cc1OCCCN1CCC[C@H]1CO Show InChI InChI=1S/C29H33N7O5S/c1-40-24-13-21-23(14-25(24)41-11-5-10-36-9-4-6-18(36)16-37)32-17-33-28(21)35-29-31-15-19(42-29)12-26(38)34-22-8-3-2-7-20(22)27(30)39/h2-3,7-8,13-15,17-18,37H,4-6,9-12,16H2,1H3,(H2,30,39)(H,34,38)(H,31,32,33,35)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 452 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181534

(CHEMBL202502 | N-(3-fluorophenyl)-2-(2-{[6-methoxy...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)o3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C27H29FN6O5/c1-36-23-14-21-22(15-24(23)38-9-3-6-34-7-10-37-11-8-34)30-17-31-26(21)33-27-29-16-20(39-27)13-25(35)32-19-5-2-4-18(28)12-19/h2,4-5,12,14-17H,3,6-11,13H2,1H3,(H,32,35)(H,29,30,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 472 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181549
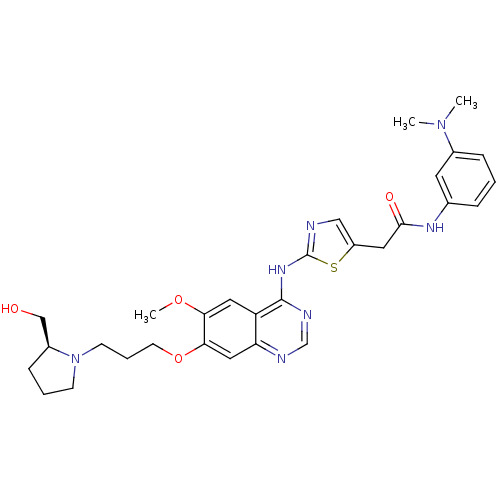
(CHEMBL203422 | N-[3-(dimethylamino)phenyl]-2-{2-[(...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(c4)N(C)C)s3)ncnc2cc1OCCCN1CCC[C@H]1CO Show InChI InChI=1S/C30H37N7O4S/c1-36(2)21-8-4-7-20(13-21)34-28(39)14-23-17-31-30(42-23)35-29-24-15-26(40-3)27(16-25(24)32-19-33-29)41-12-6-11-37-10-5-9-22(37)18-38/h4,7-8,13,15-17,19,22,38H,5-6,9-12,14,18H2,1-3H3,(H,34,39)(H,31,32,33,35)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 483 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181539
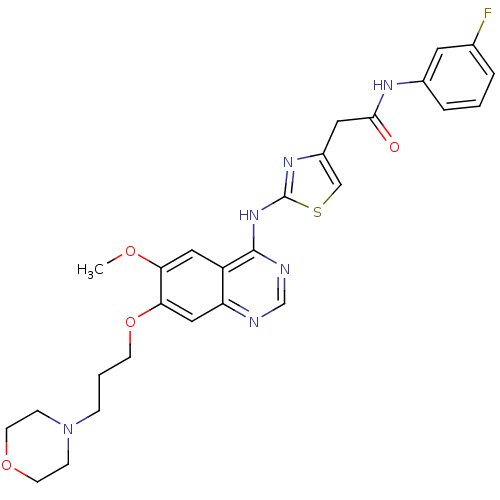
(CHEMBL382804 | N-(3-fluorophenyl)-2-(2-{[6-methoxy...)Show SMILES COc1cc2c(Nc3nc(CC(=O)Nc4cccc(F)c4)cs3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C27H29FN6O4S/c1-36-23-14-21-22(15-24(23)38-9-3-6-34-7-10-37-11-8-34)29-17-30-26(21)33-27-32-20(16-39-27)13-25(35)31-19-5-2-4-18(28)12-19/h2,4-5,12,14-17H,3,6-11,13H2,1H3,(H,31,35)(H,29,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 578 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against EGFR |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50181553
(CHEMBL202370 | N-(4-fluorophenyl)-2-{[6-methoxy-7-...)Show SMILES COc1cc2c(Nc3ncc([nH]3)C(=O)Nc3ccc(F)cc3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C26H28FN7O4/c1-36-22-13-19-20(14-23(22)38-10-2-7-34-8-11-37-12-9-34)29-16-30-24(19)33-26-28-15-21(32-26)25(35)31-18-5-3-17(27)4-6-18/h3-6,13-16H,2,7-12H2,1H3,(H,31,35)(H2,28,29,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
In vitro inhibition constant for Aurora-A |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against KDR |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against SRC |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against FAK |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1/2/3/4
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against FGFR |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM14210

(4-(4-(N-benzoylamino)anilino)-6-methoxy-7-(3-(1-mo...)Show SMILES COc1cc2c(Nc3ccc(NC(=O)c4ccccc4)cc3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C29H31N5O4/c1-36-26-18-24-25(19-27(26)38-15-5-12-34-13-16-37-17-14-34)30-20-31-28(24)32-22-8-10-23(11-9-22)33-29(35)21-6-3-2-4-7-21/h2-4,6-11,18-20H,5,12-17H2,1H3,(H,33,35)(H,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of in vitro CDK1 kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM14210

(4-(4-(N-benzoylamino)anilino)-6-methoxy-7-(3-(1-mo...)Show SMILES COc1cc2c(Nc3ccc(NC(=O)c4ccccc4)cc3)ncnc2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C29H31N5O4/c1-36-26-18-24-25(19-27(26)38-15-5-12-34-13-16-37-17-14-34)30-20-31-28(24)32-22-8-10-23(11-9-22)33-29(35)21-6-3-2-4-7-21/h2-4,6-11,18-20H,5,12-17H2,1H3,(H,33,35)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of in vitro Plk1 kinase activity |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against human hERG receptor |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against IGFR |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against CSK |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against JAK3 |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against CDK2 |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against p38-alpha MAPK |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against PKA |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50181532

(CHEMBL201307 | N-(3-fluorophenyl)-2-(2-(7-(3-(4-(h...)Show SMILES COc1cc2c(Nc3ncc(CC(=O)Nc4cccc(F)c4)s3)ncnc2cc1OCCCN1CCC(CO)CC1 Show InChI InChI=1S/C29H33FN6O4S/c1-39-25-14-23-24(15-26(25)40-11-3-8-36-9-6-19(17-37)7-10-36)32-18-33-28(23)35-29-31-16-22(41-29)13-27(38)34-21-5-2-4-20(30)12-21/h2,4-5,12,14-16,18-19,37H,3,6-11,13,17H2,1H3,(H,34,38)(H,31,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibitory activity against JNK1alpha |
J Med Chem 49: 955-70 (2006)
Article DOI: 10.1021/jm050786h
BindingDB Entry DOI: 10.7270/Q24J0FXV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data