 Found 67 hits of Enzyme Inhibition Constant Data
Found 67 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186291
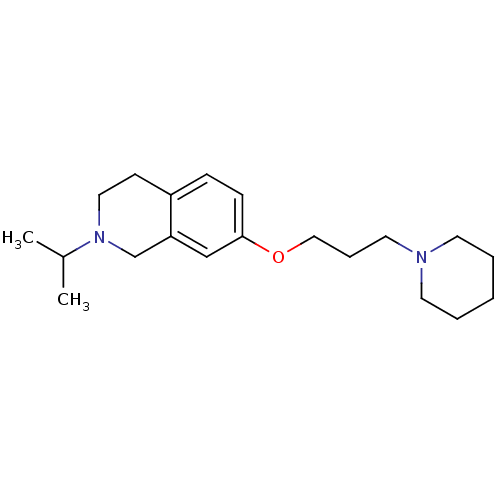
(2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-...)Show InChI InChI=1S/C20H32N2O/c1-17(2)22-13-9-18-7-8-20(15-19(18)16-22)23-14-6-12-21-10-4-3-5-11-21/h7-8,15,17H,3-6,9-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186278
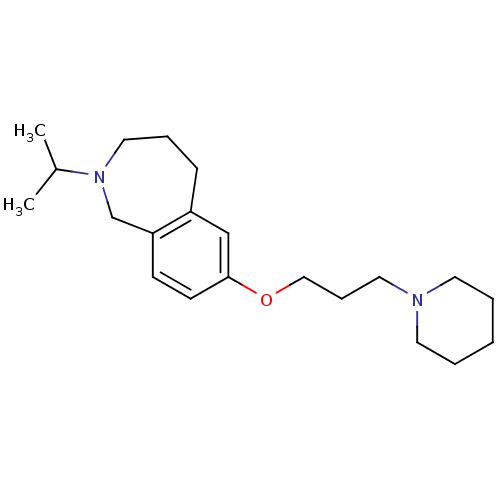
(2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...)Show InChI InChI=1S/C21H34N2O/c1-18(2)23-14-6-8-19-16-21(10-9-20(19)17-23)24-15-7-13-22-11-4-3-5-12-22/h9-10,16,18H,3-8,11-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186270
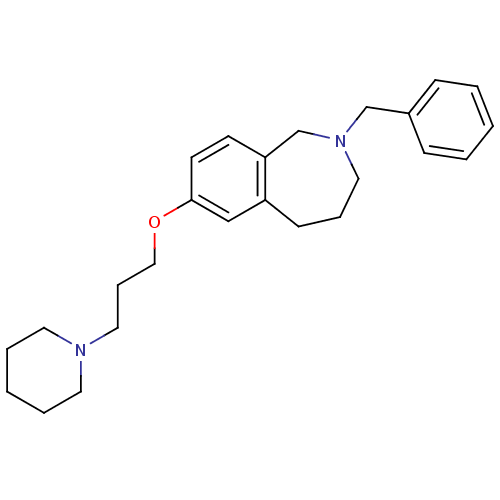
(2-benzyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tet...)Show InChI InChI=1S/C25H34N2O/c1-3-9-22(10-4-1)20-27-16-7-11-23-19-25(13-12-24(23)21-27)28-18-8-17-26-14-5-2-6-15-26/h1,3-4,9-10,12-13,19H,2,5-8,11,14-18,20-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186290
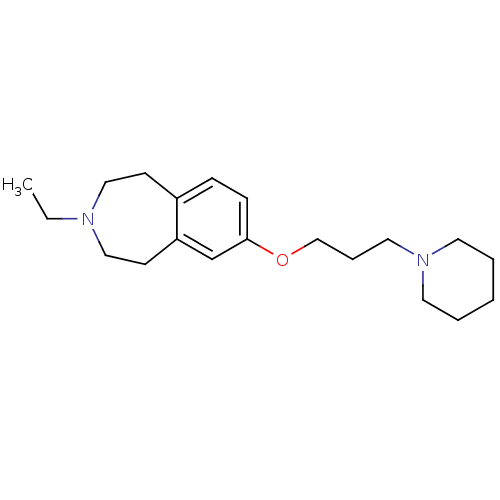
(3-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...)Show InChI InChI=1S/C20H32N2O/c1-2-21-14-9-18-7-8-20(17-19(18)10-15-21)23-16-6-13-22-11-4-3-5-12-22/h7-8,17H,2-6,9-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186269
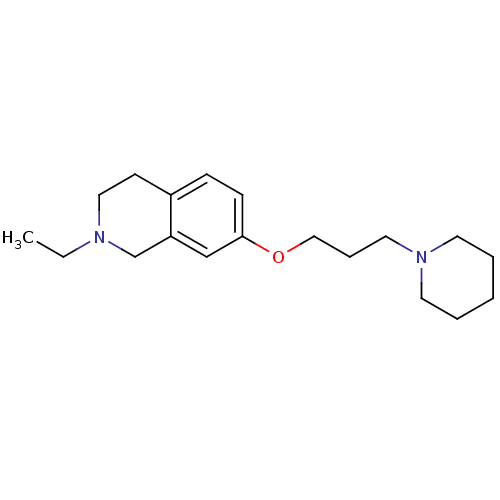
(2-ethyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-20-13-9-17-7-8-19(15-18(17)16-20)22-14-6-12-21-10-4-3-5-11-21/h7-8,15H,2-6,9-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186309

(2-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...)Show InChI InChI=1S/C20H32N2O/c1-2-21-13-6-8-18-16-20(10-9-19(18)17-21)23-15-7-14-22-11-4-3-5-12-22/h9-10,16H,2-8,11-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186292

(2-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-8-21(9-4-1)19-26-16-12-22-10-11-24(18-23(22)20-26)27-17-7-15-25-13-5-2-6-14-25/h10-11,18,21H,1-9,12-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186292

(2-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-8-21(9-4-1)19-26-16-12-22-10-11-24(18-23(22)20-26)27-17-7-15-25-13-5-2-6-14-25/h10-11,18,21H,1-9,12-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186306
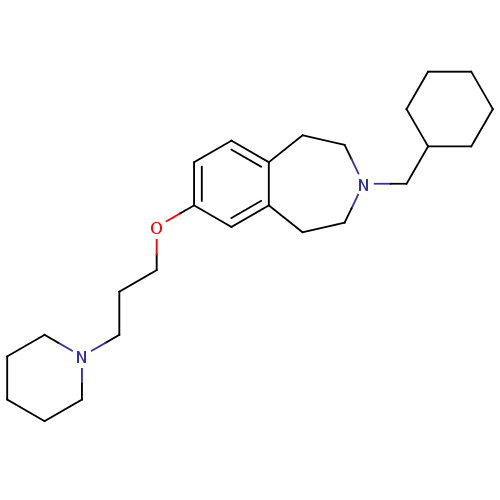
(3-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C25H40N2O/c1-3-8-22(9-4-1)21-27-17-12-23-10-11-25(20-24(23)13-18-27)28-19-7-16-26-14-5-2-6-15-26/h10-11,20,22H,1-9,12-19,21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186316
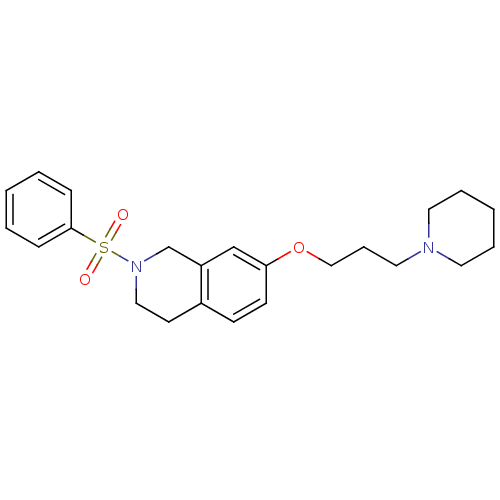
(2-(phenylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-1...)Show SMILES O=S(=O)(N1CCc2ccc(OCCCN3CCCCC3)cc2C1)c1ccccc1 Show InChI InChI=1S/C23H30N2O3S/c26-29(27,23-8-3-1-4-9-23)25-16-12-20-10-11-22(18-21(20)19-25)28-17-7-15-24-13-5-2-6-14-24/h1,3-4,8-11,18H,2,5-7,12-17,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186283
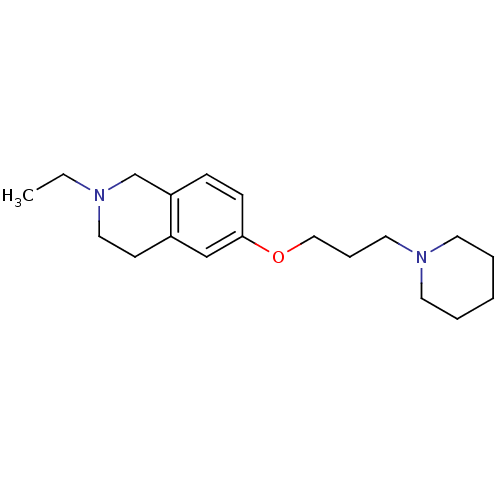
(2-ethyl-6-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-20-13-9-17-15-19(8-7-18(17)16-20)22-14-6-12-21-10-4-3-5-11-21/h7-8,15H,2-6,9-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186309

(2-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...)Show InChI InChI=1S/C20H32N2O/c1-2-21-13-6-8-18-16-20(10-9-19(18)17-21)23-15-7-14-22-11-4-3-5-12-22/h9-10,16H,2-8,11-15,17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186293
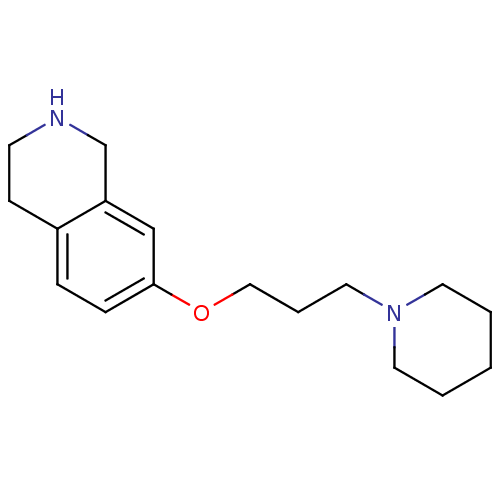
(7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetrahydrois...)Show InChI InChI=1S/C17H26N2O/c1-2-9-19(10-3-1)11-4-12-20-17-6-5-15-7-8-18-14-16(15)13-17/h5-6,13,18H,1-4,7-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186269

(2-ethyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-20-13-9-17-7-8-19(15-18(17)16-20)22-14-6-12-21-10-4-3-5-11-21/h7-8,15H,2-6,9-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186311
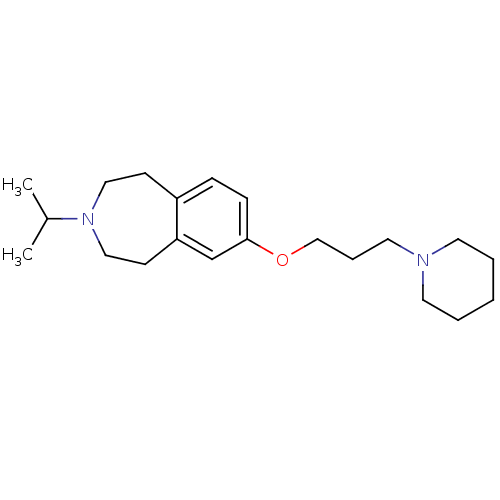
(3-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...)Show InChI InChI=1S/C21H34N2O/c1-18(2)23-14-9-19-7-8-21(17-20(19)10-15-23)24-16-6-13-22-11-4-3-5-12-22/h7-8,17-18H,3-6,9-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186270

(2-benzyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tet...)Show InChI InChI=1S/C25H34N2O/c1-3-9-22(10-4-1)20-27-16-7-11-23-19-25(13-12-24(23)21-27)28-18-8-17-26-14-5-2-6-15-26/h1,3-4,9-10,12-13,19H,2,5-8,11,14-18,20-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186291

(2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-...)Show InChI InChI=1S/C20H32N2O/c1-17(2)22-13-9-18-7-8-20(15-19(18)16-22)23-14-6-12-21-10-4-3-5-11-21/h7-8,15,17H,3-6,9-14,16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186306

(3-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C25H40N2O/c1-3-8-22(9-4-1)21-27-17-12-23-10-11-25(20-24(23)13-18-27)28-19-7-16-26-14-5-2-6-15-26/h10-11,20,22H,1-9,12-19,21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186296
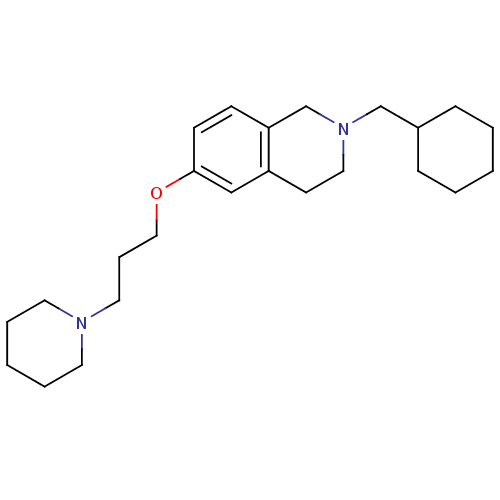
(2-(cyclohexylmethyl)-6-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-8-21(9-4-1)19-26-16-12-22-18-24(11-10-23(22)20-26)27-17-7-15-25-13-5-2-6-14-25/h10-11,18,21H,1-9,12-17,19-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186290

(3-ethyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-tetr...)Show InChI InChI=1S/C20H32N2O/c1-2-21-14-9-18-7-8-20(17-19(18)10-15-21)23-16-6-13-22-11-4-3-5-12-22/h7-8,17H,2-6,9-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186278

(2-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...)Show InChI InChI=1S/C21H34N2O/c1-18(2)23-14-6-8-19-16-21(10-9-20(19)17-23)24-15-7-13-22-11-4-3-5-12-22/h9-10,16,18H,3-8,11-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186311

(3-isopropyl-7-(3-(piperidin-1-yl)propoxy)-2,3,4,5-...)Show InChI InChI=1S/C21H34N2O/c1-18(2)23-14-9-19-7-8-21(17-20(19)10-15-23)24-16-6-13-22-11-4-3-5-12-22/h7-8,17-18H,3-6,9-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186281
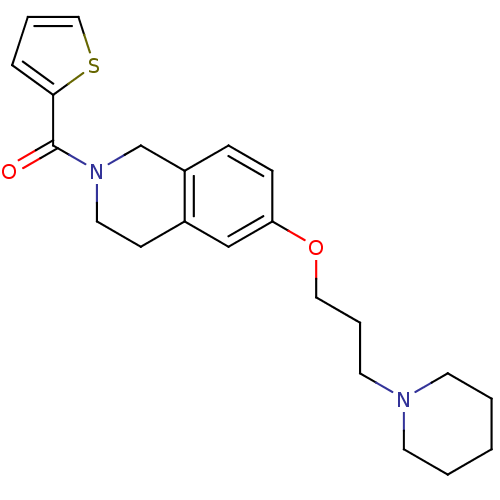
((6-(3-(piperidin-1-yl)propoxy)-3,4-dihydroisoquino...)Show InChI InChI=1S/C22H28N2O2S/c25-22(21-6-4-15-27-21)24-13-9-18-16-20(8-7-19(18)17-24)26-14-5-12-23-10-2-1-3-11-23/h4,6-8,15-16H,1-3,5,9-14,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186295
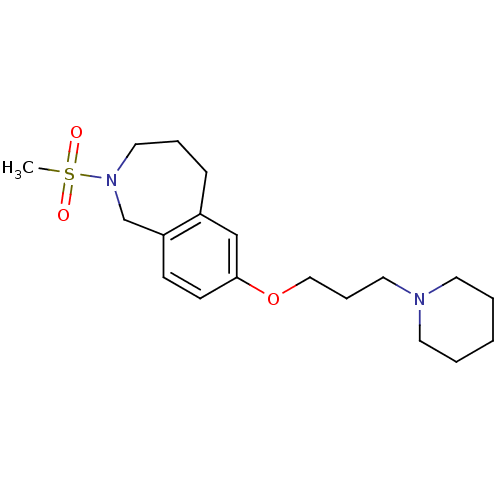
(2-(methylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-2...)Show InChI InChI=1S/C19H30N2O3S/c1-25(22,23)21-13-5-7-17-15-19(9-8-18(17)16-21)24-14-6-12-20-10-3-2-4-11-20/h8-9,15H,2-7,10-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186285

(CHEMBL208829 | N-isopropyl-7-(3-(piperidin-1-yl)pr...)Show InChI InChI=1S/C21H33N3O2/c1-17(2)22-21(25)24-13-9-18-7-8-20(15-19(18)16-24)26-14-6-12-23-10-4-3-5-11-23/h7-8,15,17H,3-6,9-14,16H2,1-2H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186286

(1-(phenylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-1...)Show SMILES O=S(=O)(N1CCCc2ccc(OCCCN3CCCCC3)cc12)c1ccccc1 Show InChI InChI=1S/C23H30N2O3S/c26-29(27,22-10-3-1-4-11-22)25-17-7-9-20-12-13-21(19-23(20)25)28-18-8-16-24-14-5-2-6-15-24/h1,3-4,10-13,19H,2,5-9,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186305

(2-(phenylsulfonyl)-6-(3-(piperidin-1-yl)propoxy)-1...)Show SMILES O=S(=O)(N1CCc2cc(OCCCN3CCCCC3)ccc2C1)c1ccccc1 Show InChI InChI=1S/C23H30N2O3S/c26-29(27,23-8-3-1-4-9-23)25-16-12-20-18-22(11-10-21(20)19-25)28-17-7-15-24-13-5-2-6-14-24/h1,3-4,8-11,18H,2,5-7,12-17,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186273
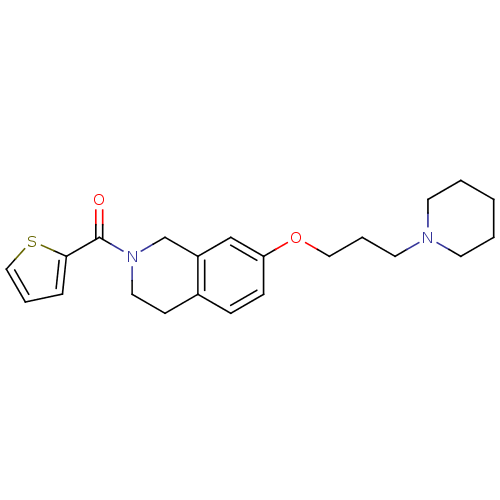
((7-(3-(piperidin-1-yl)propoxy)-3,4-dihydroisoquino...)Show InChI InChI=1S/C22H28N2O2S/c25-22(21-6-4-15-27-21)24-13-9-18-7-8-20(16-19(18)17-24)26-14-5-12-23-10-2-1-3-11-23/h4,6-8,15-16H,1-3,5,9-14,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186300
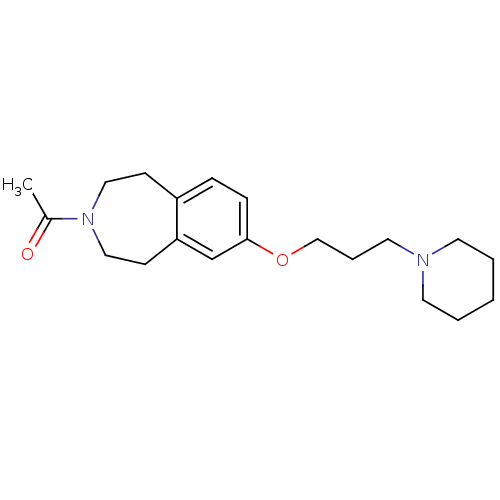
(1-(7-(3-(piperidin-1-yl)propoxy)-1,2,4,5-tetrahydr...)Show InChI InChI=1S/C20H30N2O2/c1-17(23)22-13-8-18-6-7-20(16-19(18)9-14-22)24-15-5-12-21-10-3-2-4-11-21/h6-7,16H,2-5,8-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186275
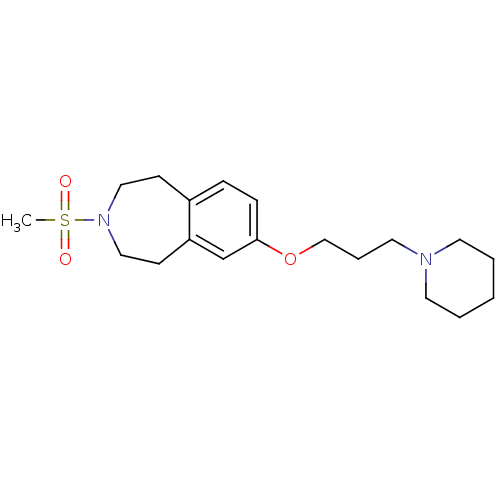
(3-(methylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-2...)Show InChI InChI=1S/C19H30N2O3S/c1-25(22,23)21-13-8-17-6-7-19(16-18(17)9-14-21)24-15-5-12-20-10-3-2-4-11-20/h6-7,16H,2-5,8-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186284

(2-(cyclohexylmethyl)-5-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-9-21(10-4-1)19-26-17-13-23-22(20-26)11-7-12-24(23)27-18-8-16-25-14-5-2-6-15-25/h7,11-12,21H,1-6,8-10,13-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186297

(CHEMBL209099 | N-isopropyl-7-(3-(piperidin-1-yl)pr...)Show InChI InChI=1S/C22H35N3O2/c1-18(2)23-22(26)25-14-6-8-19-16-21(10-9-20(19)17-25)27-15-7-13-24-11-4-3-5-12-24/h9-10,16,18H,3-8,11-15,17H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 17.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186289

(1-(phenylsulfonyl)-6-(3-(piperidin-1-yl)propoxy)-1...)Show SMILES O=S(=O)(N1CCCc2cc(OCCCN3CCCCC3)ccc12)c1ccccc1 Show InChI InChI=1S/C23H30N2O3S/c26-29(27,22-10-3-1-4-11-22)25-17-7-9-20-19-21(12-13-23(20)25)28-18-8-16-24-14-5-2-6-15-24/h1,3-4,10-13,19H,2,5-9,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186277

(1-(7-(3-(piperidin-1-yl)propoxy)-3,4-dihydroisoqui...)Show InChI InChI=1S/C19H28N2O2/c1-16(22)21-12-8-17-6-7-19(14-18(17)15-21)23-13-5-11-20-9-3-2-4-10-20/h6-7,14H,2-5,8-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186279
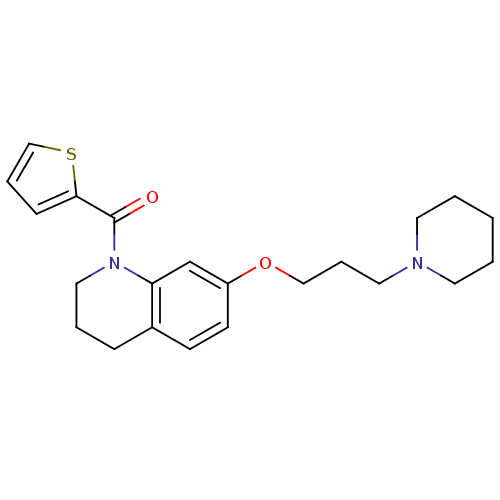
((7-(3-(piperidin-1-yl)propoxy)-3,4-dihydroquinolin...)Show InChI InChI=1S/C22H28N2O2S/c25-22(21-8-5-16-27-21)24-14-4-7-18-9-10-19(17-20(18)24)26-15-6-13-23-11-2-1-3-12-23/h5,8-10,16-17H,1-4,6-7,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186288

(2-(methylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-1...)Show InChI InChI=1S/C18H28N2O3S/c1-24(21,22)20-12-8-16-6-7-18(14-17(16)15-20)23-13-5-11-19-9-3-2-4-10-19/h6-7,14H,2-5,8-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186268
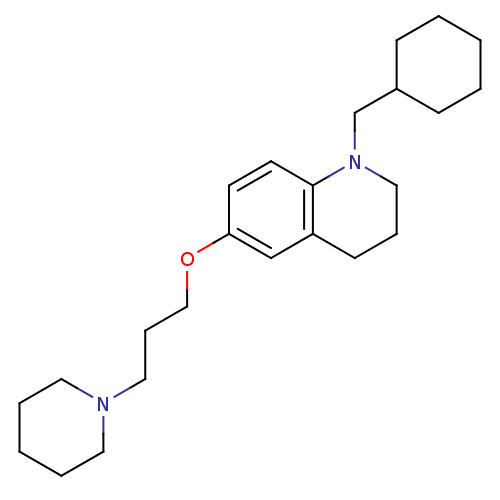
(1-(cyclohexylmethyl)-6-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-9-21(10-4-1)20-26-17-7-11-22-19-23(12-13-24(22)26)27-18-8-16-25-14-5-2-6-15-25/h12-13,19,21H,1-11,14-18,20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186297

(CHEMBL209099 | N-isopropyl-7-(3-(piperidin-1-yl)pr...)Show InChI InChI=1S/C22H35N3O2/c1-18(2)23-22(26)25-14-6-8-19-16-21(10-9-20(19)17-25)27-15-7-13-24-11-4-3-5-12-24/h9-10,16,18H,3-8,11-15,17H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 32.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186277

(1-(7-(3-(piperidin-1-yl)propoxy)-3,4-dihydroisoqui...)Show InChI InChI=1S/C19H28N2O2/c1-16(22)21-12-8-17-6-7-19(14-18(17)15-21)23-13-5-11-20-9-3-2-4-10-20/h6-7,14H,2-5,8-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186275

(3-(methylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-2...)Show InChI InChI=1S/C19H30N2O3S/c1-25(22,23)21-13-8-17-6-7-19(16-18(17)9-14-21)24-15-5-12-20-10-3-2-4-11-20/h6-7,16H,2-5,8-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 38.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186285

(CHEMBL208829 | N-isopropyl-7-(3-(piperidin-1-yl)pr...)Show InChI InChI=1S/C21H33N3O2/c1-17(2)22-21(25)24-13-9-18-7-8-20(15-19(18)16-24)26-14-6-12-23-10-4-3-5-11-23/h7-8,15,17H,3-6,9-14,16H2,1-2H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186312

(1-ethyl-6-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-21-14-6-8-17-16-18(9-10-19(17)21)22-15-7-13-20-11-4-3-5-12-20/h9-10,16H,2-8,11-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186287

(CHEMBL377975 | N-isopropyl-7-(3-(piperidin-1-yl)pr...)Show InChI InChI=1S/C22H35N3O2/c1-18(2)23-22(26)25-14-9-19-7-8-21(17-20(19)10-15-25)27-16-6-13-24-11-4-3-5-12-24/h7-8,17-18H,3-6,9-16H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186300

(1-(7-(3-(piperidin-1-yl)propoxy)-1,2,4,5-tetrahydr...)Show InChI InChI=1S/C20H30N2O2/c1-17(23)22-13-8-18-6-7-20(16-19(18)9-14-22)24-15-5-12-21-10-3-2-4-11-21/h6-7,16H,2-5,8-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186272

(6-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetrahydroqu...)Show InChI InChI=1S/C17H26N2O/c1-2-10-19(11-3-1)12-5-13-20-16-7-8-17-15(14-16)6-4-9-18-17/h7-8,14,18H,1-6,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186307
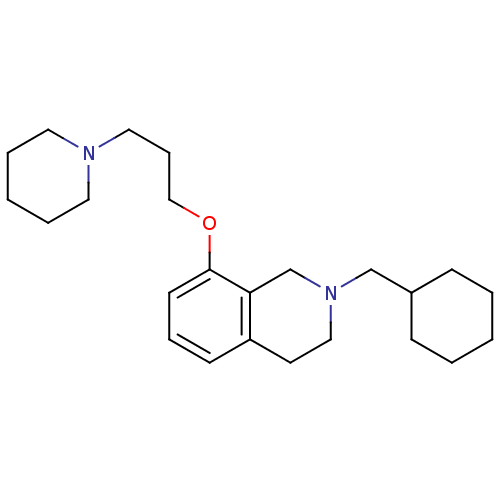
(2-(cyclohexylmethyl)-8-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-9-21(10-4-1)19-26-17-13-22-11-7-12-24(23(22)20-26)27-18-8-16-25-14-5-2-6-15-25/h7,11-12,21H,1-6,8-10,13-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186295

(2-(methylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-2...)Show InChI InChI=1S/C19H30N2O3S/c1-25(22,23)21-13-5-7-17-15-19(9-8-18(17)16-21)24-14-6-12-20-10-3-2-4-11-20/h8-9,15H,2-7,10-14,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 70.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50186288

(2-(methylsulfonyl)-7-(3-(piperidin-1-yl)propoxy)-1...)Show InChI InChI=1S/C18H28N2O3S/c1-24(21,22)20-12-8-16-6-7-18(14-17(16)15-20)23-13-5-11-19-9-3-2-4-10-19/h6-7,14H,2-5,8-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 74.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from rat cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186276

(2-ethyl-5-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-20-14-10-18-17(16-20)8-6-9-19(18)22-15-7-13-21-11-4-3-5-12-21/h6,8-9H,2-5,7,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 84.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186308
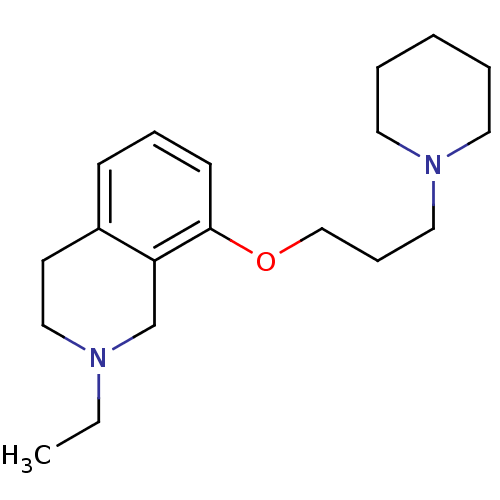
(2-ethyl-8-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-20-14-10-17-8-6-9-19(18(17)16-20)22-15-7-13-21-11-4-3-5-12-21/h6,8-9H,2-5,7,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 95.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186314

(7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetrahydroqu...)Show InChI InChI=1S/C17H26N2O/c1-2-10-19(11-3-1)12-5-13-20-16-8-7-15-6-4-9-18-17(15)14-16/h7-8,14,18H,1-6,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186313

(1-(cyclohexylmethyl)-5-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-10-21(11-4-1)20-26-18-8-12-22-23(26)13-7-14-24(22)27-19-9-17-25-15-5-2-6-16-25/h7,13-14,21H,1-6,8-12,15-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186315

(8-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetrahydrois...)Show InChI InChI=1S/C17H26N2O/c1-2-10-19(11-3-1)12-5-13-20-17-7-4-6-15-8-9-18-14-16(15)17/h4,6-7,18H,1-3,5,8-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186299

(1-ethyl-7-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-21-14-6-8-17-9-10-18(16-19(17)21)22-15-7-13-20-11-4-3-5-12-20/h9-10,16H,2-8,11-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186318

(1-(cyclohexylmethyl)-7-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-9-21(10-4-1)20-26-17-7-11-22-12-13-23(19-24(22)26)27-18-8-16-25-14-5-2-6-15-25/h12-13,19,21H,1-11,14-18,20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186304

(5-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetrahydroqu...)Show InChI InChI=1S/C17H26N2O/c1-2-11-19(12-3-1)13-6-14-20-17-9-4-8-16-15(17)7-5-10-18-16/h4,8-9,18H,1-3,5-7,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186274

(5-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetrahydrois...)Show InChI InChI=1S/C17H26N2O/c1-2-10-19(11-3-1)12-5-13-20-17-7-4-6-15-14-18-9-8-16(15)17/h4,6-7,18H,1-3,5,8-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186271
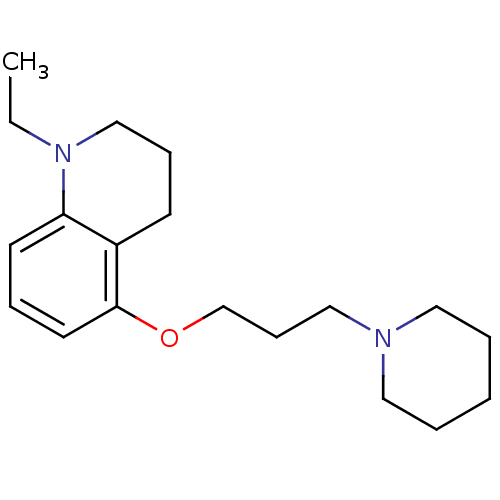
(1-ethyl-5-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-21-15-7-9-17-18(21)10-6-11-19(17)22-16-8-14-20-12-4-3-5-13-20/h6,10-11H,2-5,7-9,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186302

((8-(3-(piperidin-1-yl)propoxy)-3,4-dihydroquinolin...)Show InChI InChI=1S/C22H28N2O2S/c25-22(20-11-6-17-27-20)24-15-5-9-18-8-4-10-19(21(18)24)26-16-7-14-23-12-2-1-3-13-23/h4,6,8,10-11,17H,1-3,5,7,9,12-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186303

(8-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetrahydroqu...)Show InChI InChI=1S/C17H26N2O/c1-2-11-19(12-3-1)13-6-14-20-16-9-4-7-15-8-5-10-18-17(15)16/h4,7,9,18H,1-3,5-6,8,10-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186294

(1-(cyclohexylmethyl)-8-(3-(piperidin-1-yl)propoxy)...)Show InChI InChI=1S/C24H38N2O/c1-3-10-21(11-4-1)20-26-18-8-13-22-12-7-14-23(24(22)26)27-19-9-17-25-15-5-2-6-16-25/h7,12,14,21H,1-6,8-11,13,15-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186317
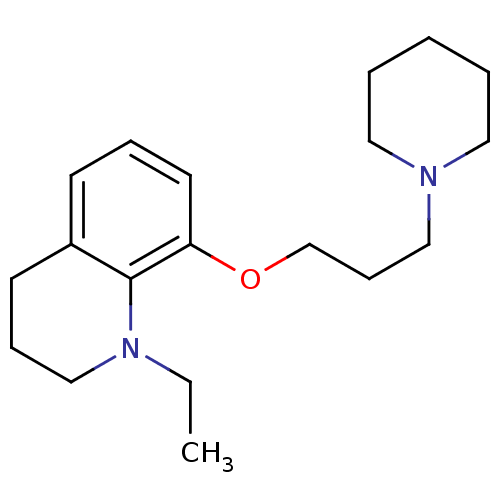
(1-ethyl-8-(3-(piperidin-1-yl)propoxy)-1,2,3,4-tetr...)Show InChI InChI=1S/C19H30N2O/c1-2-21-15-7-10-17-9-6-11-18(19(17)21)22-16-8-14-20-12-4-3-5-13-20/h6,9,11H,2-5,7-8,10,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186298

(2-(phenylsulfonyl)-5-(3-(piperidin-1-yl)propoxy)-1...)Show SMILES O=S(=O)(N1CCc2c(C1)cccc2OCCCN1CCCCC1)c1ccccc1 Show InChI InChI=1S/C23H30N2O3S/c26-29(27,21-10-3-1-4-11-21)25-17-13-22-20(19-25)9-7-12-23(22)28-18-8-16-24-14-5-2-6-15-24/h1,3-4,7,9-12H,2,5-6,8,13-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186310
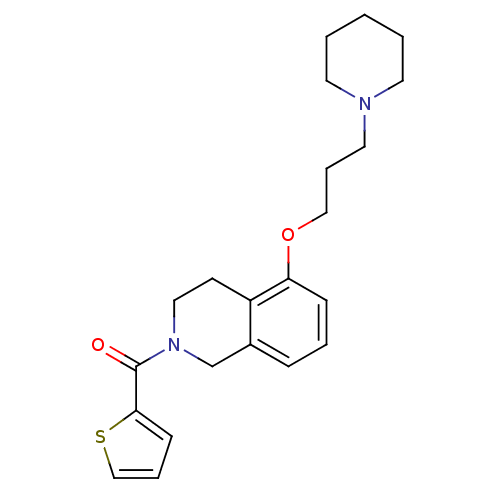
((5-(3-(piperidin-1-yl)propoxy)-3,4-dihydroisoquino...)Show InChI InChI=1S/C22H28N2O2S/c25-22(21-9-5-16-27-21)24-14-10-19-18(17-24)7-4-8-20(19)26-15-6-13-23-11-2-1-3-12-23/h4-5,7-9,16H,1-3,6,10-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186301
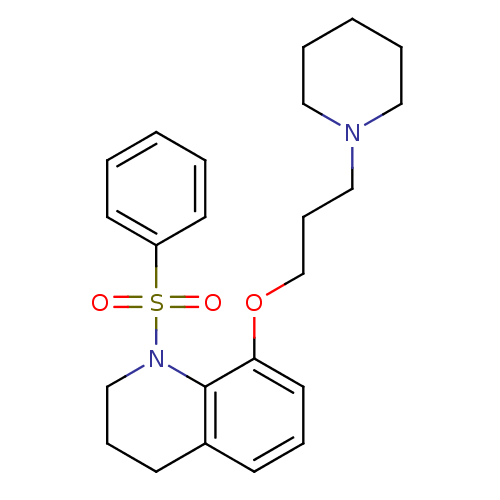
(1-(phenylsulfonyl)-8-(3-(piperidin-1-yl)propoxy)-1...)Show SMILES O=S(=O)(N1CCCc2cccc(OCCCN3CCCCC3)c12)c1ccccc1 Show InChI InChI=1S/C23H30N2O3S/c26-29(27,21-12-3-1-4-13-21)25-18-8-11-20-10-7-14-22(23(20)25)28-19-9-17-24-15-5-2-6-16-24/h1,3-4,7,10,12-14H,2,5-6,8-9,11,15-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186280
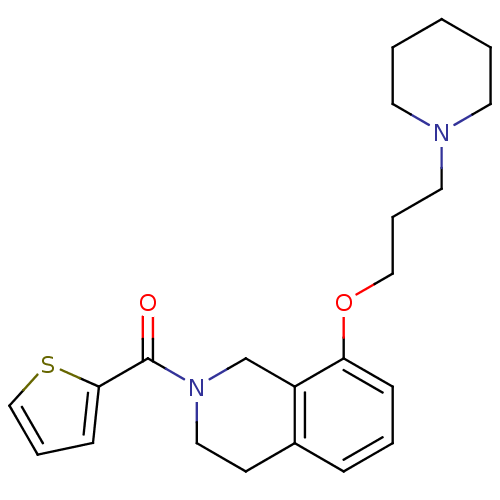
((8-(3-(piperidin-1-yl)propoxy)-3,4-dihydroisoquino...)Show InChI InChI=1S/C22H28N2O2S/c25-22(21-9-5-16-27-21)24-14-10-18-7-4-8-20(19(18)17-24)26-15-6-13-23-11-2-1-3-12-23/h4-5,7-9,16H,1-3,6,10-15,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50186282

(2-(phenylsulfonyl)-8-(3-(piperidin-1-yl)propoxy)-1...)Show SMILES O=S(=O)(N1CCc2cccc(OCCCN3CCCCC3)c2C1)c1ccccc1 Show InChI InChI=1S/C23H30N2O3S/c26-29(27,21-10-3-1-4-11-21)25-17-13-20-9-7-12-23(22(20)19-25)28-18-8-16-24-14-5-2-6-15-24/h1,3-4,7,9-12H,2,5-6,8,13-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from human cloned H3 receptor |
Bioorg Med Chem Lett 16: 3415-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.004
BindingDB Entry DOI: 10.7270/Q2WM1D07 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data