

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21137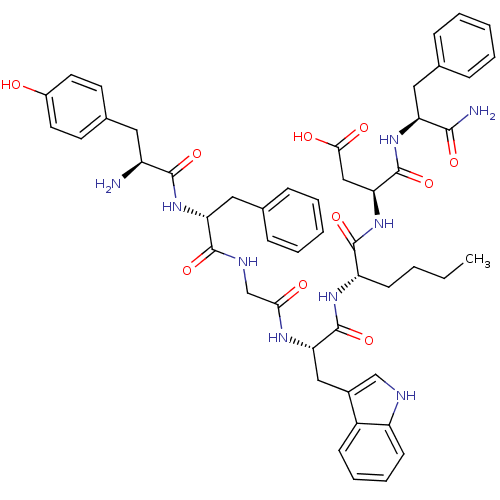 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | -55.4 | n/a | n/a | 0.900 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21135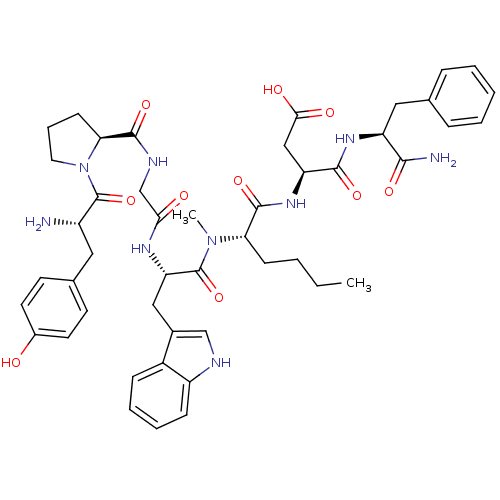 ((3S)-3-[(2S)-2-[(2S)-2-(2-{[(2S)-1-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21138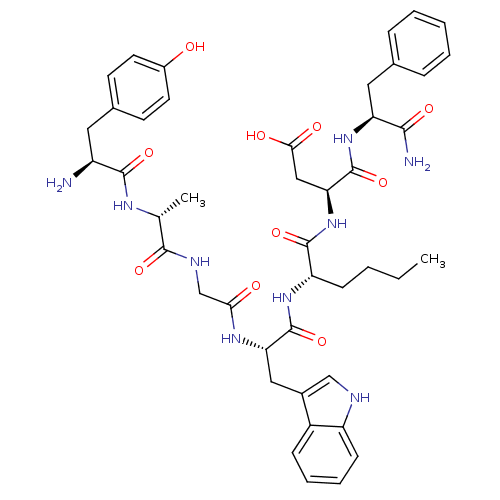 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -55.1 | n/a | n/a | 5.90 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21140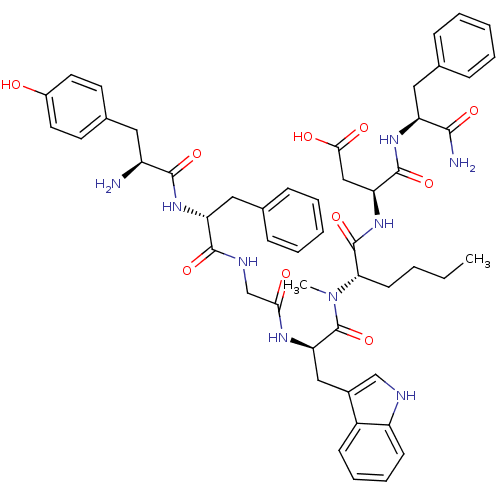 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | -53.5 | n/a | n/a | 29 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21131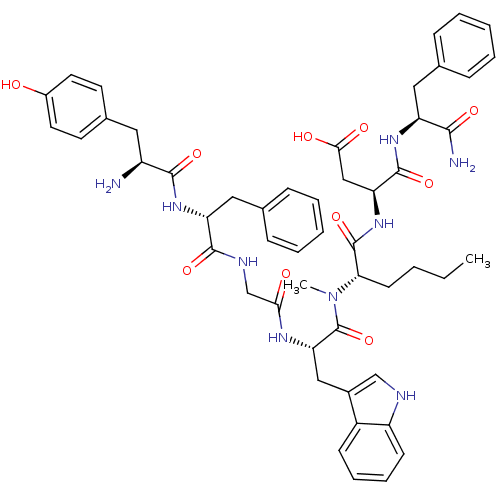 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -51.4 | n/a | n/a | 2.5 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21140 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21141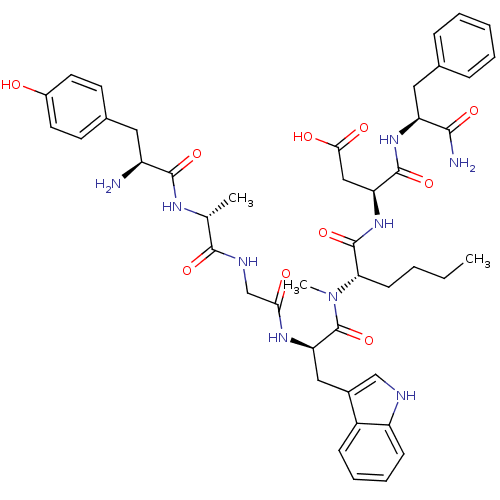 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -50.4 | n/a | n/a | 23 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21141 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21132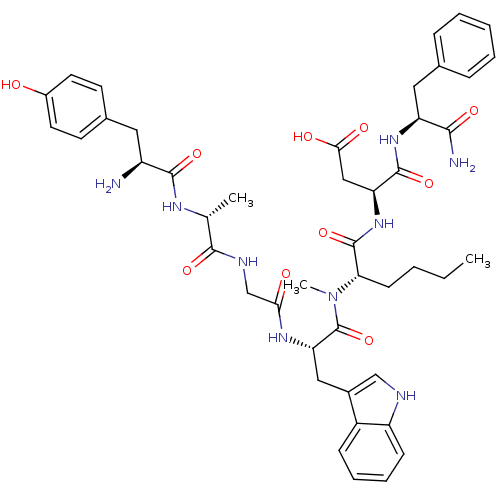 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21133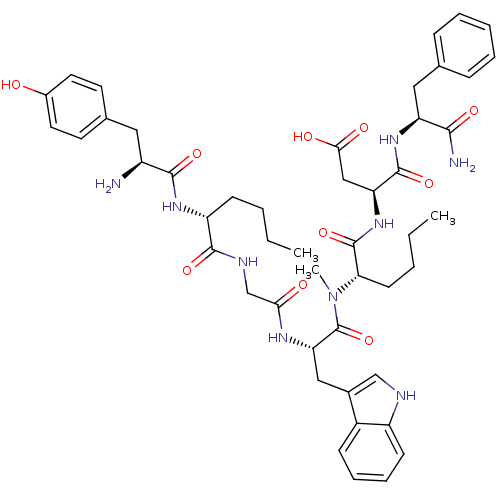 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -49.7 | n/a | n/a | 15 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21146 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21132 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -49.4 | n/a | n/a | 120 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21139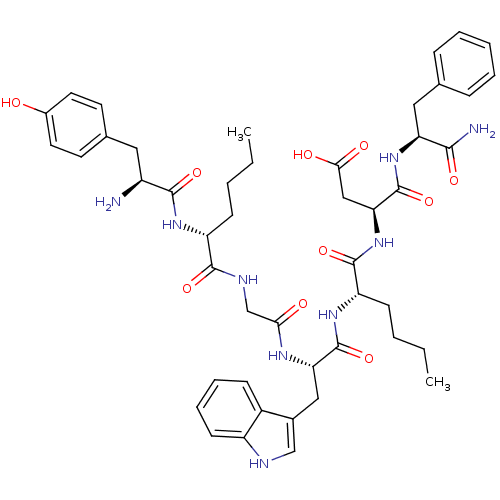 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | -49.0 | n/a | n/a | 4.5 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21134 ((3S)-3-[(2S)-2-[(2S)-2-(2-{[(2R)-1-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21133 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21136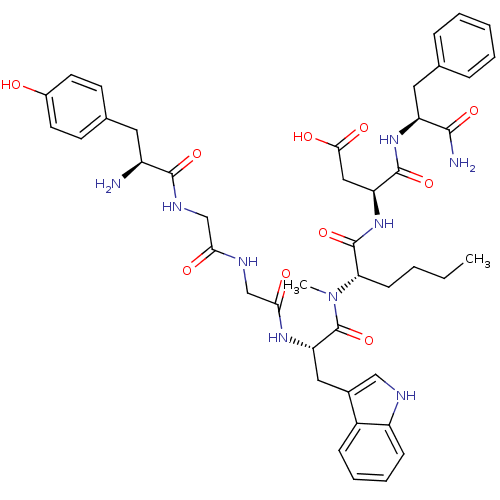 ((3S)-3-[(2S)-2-[(2S)-2-(2-{2-[(2S)-2-amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21140 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.90 | -47.0 | n/a | n/a | 530 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21138 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.80 | -46.3 | n/a | n/a | 12 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21139 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.60 | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21131 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21139 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21145 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.9 | n/a | n/a | 4.10 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21141 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | -43.7 | n/a | n/a | 38 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21133 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | -43.4 | n/a | n/a | 110 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21137 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21139 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -43.3 | n/a | n/a | 0.460 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21137 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | -42.6 | n/a | n/a | 1.40 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21132 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | -42.3 | n/a | n/a | 18 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21136 ((3S)-3-[(2S)-2-[(2S)-2-(2-{2-[(2S)-2-amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 85 | -40.4 | n/a | n/a | 76 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21134 ((3S)-3-[(2S)-2-[(2S)-2-(2-{[(2R)-1-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 85 | -40.4 | n/a | n/a | 890 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21137 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21131 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | -40.0 | n/a | n/a | 22 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21138 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21143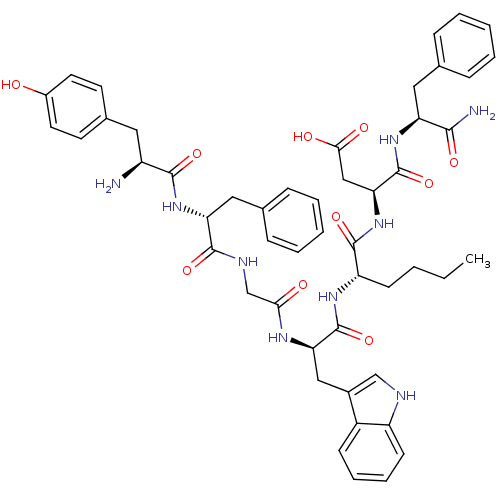 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 170 | -38.6 | n/a | n/a | 130 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21136 ((3S)-3-[(2S)-2-[(2S)-2-(2-{2-[(2S)-2-amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | -37.8 | n/a | n/a | 2 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21146 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | -37.7 | n/a | n/a | 110 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21145 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21143 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 300 | -37.2 | n/a | n/a | 45 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21142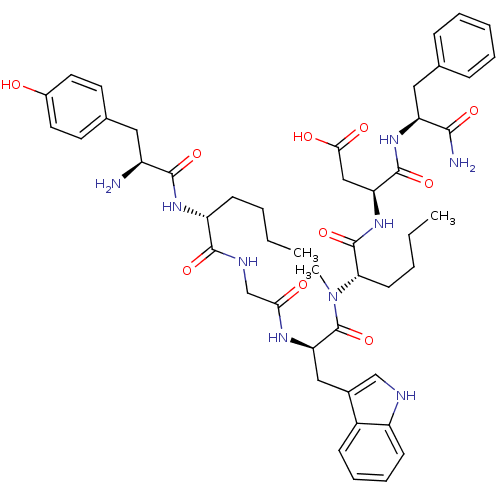 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21141 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21144 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 590 | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21144 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 860 | -34.6 | n/a | n/a | 130 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21142 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21142 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21140 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21142 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | -33.8 | n/a | n/a | 250 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21135 ((3S)-3-[(2S)-2-[(2S)-2-(2-{[(2S)-1-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21143 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21143 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21145 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21134 ((3S)-3-[(2S)-2-[(2S)-2-(2-{[(2R)-1-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | -31.9 | n/a | n/a | 900 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21144 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21146 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21131 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21132 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21146 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21138 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21144 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21134 ((3S)-3-[(2S)-2-[(2S)-2-(2-{[(2R)-1-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.5 | 1.60 | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.850 | 37 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21135 ((3S)-3-[(2S)-2-[(2S)-2-(2-{[(2S)-1-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21136 ((3S)-3-[(2S)-2-[(2S)-2-(2-{2-[(2S)-2-amino-3-(4-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.90 | 28 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21133 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21135 ((3S)-3-[(2S)-2-[(2S)-2-(2-{[(2S)-1-[(2S)-2-amino-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM21145 ((3S)-3-[(2S)-2-[(2R)-2-{2-[(2R)-2-[(2S)-2-amino-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.30 | 27 | n/a | n/a | n/a | n/a |
University of Arizona at Tucson | Assay Description Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe... | J Med Chem 49: 2868-75 (2006) Article DOI: 10.1021/jm050921q BindingDB Entry DOI: 10.7270/Q24Q7S99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||