 Found 51 hits of Enzyme Inhibition Constant Data
Found 51 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188257
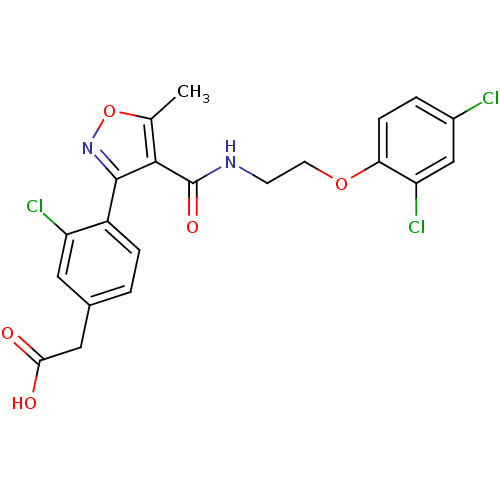
(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C21H17Cl3N2O5/c1-11-19(21(29)25-6-7-30-17-5-3-13(22)10-16(17)24)20(26-31-11)14-4-2-12(8-15(14)23)9-18(27)28/h2-5,8,10H,6-7,9H2,1H3,(H,25,29)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188255
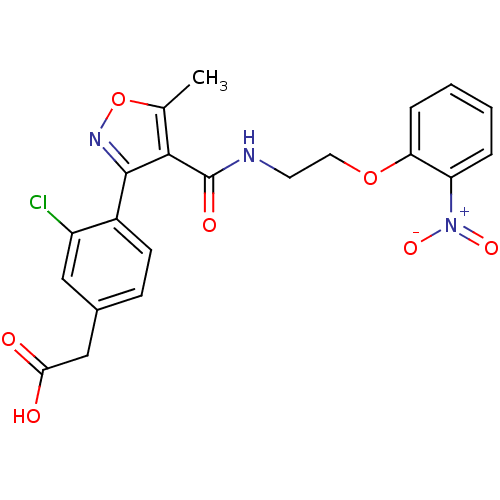
(2-(3-chloro-4-(5-methyl-4-((2-(2-nitrophenoxy)ethy...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccccc1[N+]([O-])=O)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C21H18ClN3O7/c1-12-19(20(24-32-12)14-7-6-13(10-15(14)22)11-18(26)27)21(28)23-8-9-31-17-5-3-2-4-16(17)25(29)30/h2-7,10H,8-9,11H2,1H3,(H,23,28)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188261
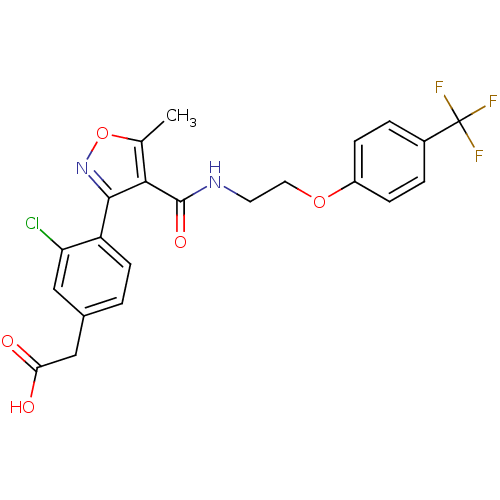
(2-(3-chloro-4-(5-methyl-4-((2-(4-(trifluoromethyl)...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(cc1)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H18ClF3N2O5/c1-12-19(20(28-33-12)16-7-2-13(10-17(16)23)11-18(29)30)21(31)27-8-9-32-15-5-3-14(4-6-15)22(24,25)26/h2-7,10H,8-9,11H2,1H3,(H,27,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM28764
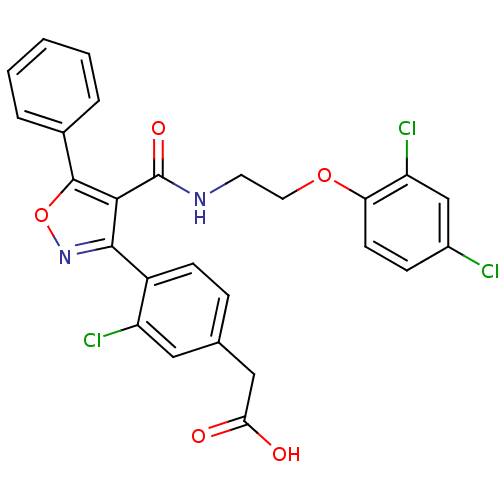
(2-[3-chloro-4-(4-{[2-(2,4-dichlorophenoxy)ethyl]ca...)Show SMILES OC(=O)Cc1ccc(-c2noc(c2C(=O)NCCOc2ccc(Cl)cc2Cl)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C26H19Cl3N2O5/c27-17-7-9-21(20(29)14-17)35-11-10-30-26(34)23-24(31-36-25(23)16-4-2-1-3-5-16)18-8-6-15(12-19(18)28)13-22(32)33/h1-9,12,14H,10-11,13H2,(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188253
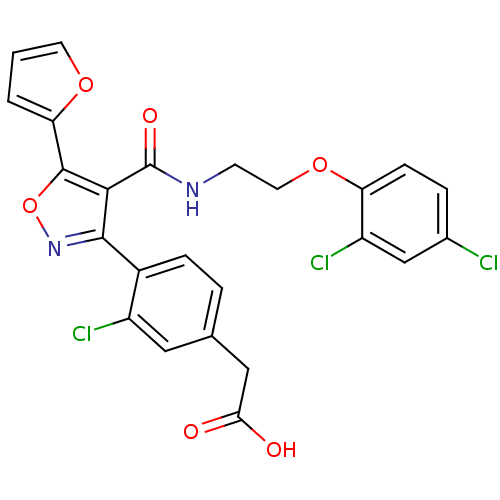
(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES OC(=O)Cc1ccc(-c2noc(-c3ccco3)c2C(=O)NCCOc2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C24H17Cl3N2O6/c25-14-4-6-18(17(27)12-14)34-9-7-28-24(32)21-22(29-35-23(21)19-2-1-8-33-19)15-5-3-13(10-16(15)26)11-20(30)31/h1-6,8,10,12H,7,9,11H2,(H,28,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188256
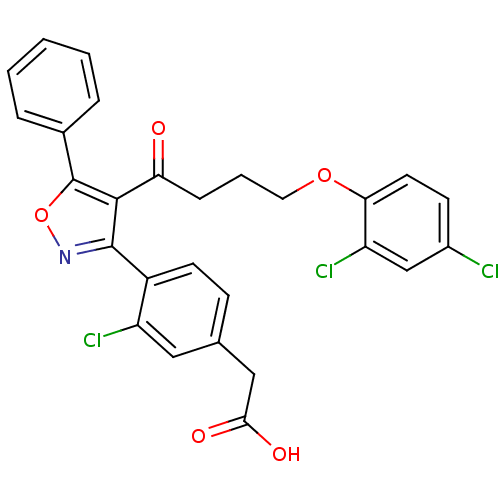
(2-(3-chloro-4-(4-(4-(2,4-dichlorophenoxy)butanoyl)...)Show SMILES OC(=O)Cc1ccc(-c2noc(c2C(=O)CCCOc2ccc(Cl)cc2Cl)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C27H20Cl3NO5/c28-18-9-11-23(21(30)15-18)35-12-4-7-22(32)25-26(31-36-27(25)17-5-2-1-3-6-17)19-10-8-16(13-20(19)29)14-24(33)34/h1-3,5-6,8-11,13,15H,4,7,12,14H2,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28661
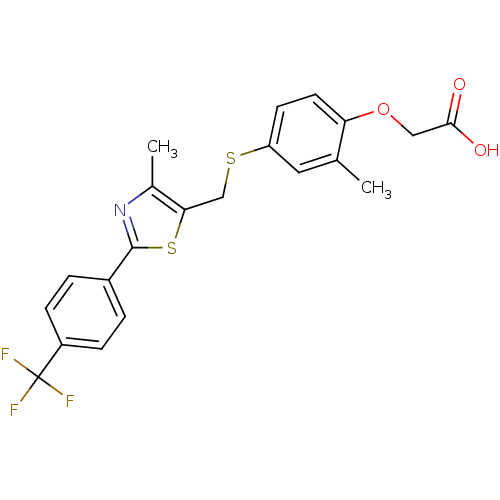
(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188259
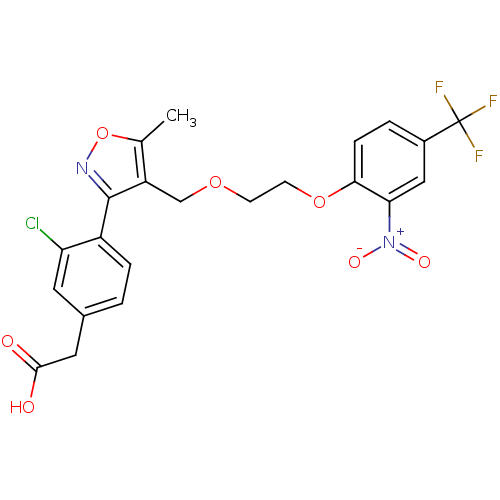
(2-(3-chloro-4-(5-methyl-4-((2-(2-nitro-4-(trifluor...)Show SMILES Cc1onc(c1COCCOc1ccc(cc1[N+]([O-])=O)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H18ClF3N2O7/c1-12-16(21(27-35-12)15-4-2-13(8-17(15)23)9-20(29)30)11-33-6-7-34-19-5-3-14(22(24,25)26)10-18(19)28(31)32/h2-5,8,10H,6-7,9,11H2,1H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188258
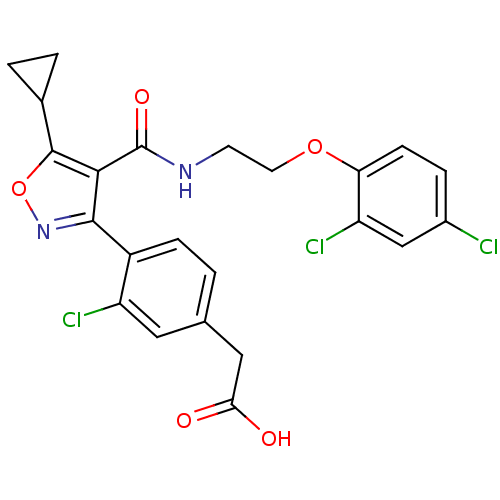
(2-(3-chloro-4-(5-cyclopropyl-4-((2-(2,4-dichloroph...)Show SMILES OC(=O)Cc1ccc(-c2noc(C3CC3)c2C(=O)NCCOc2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C23H19Cl3N2O5/c24-14-4-6-18(17(26)11-14)32-8-7-27-23(31)20-21(28-33-22(20)13-2-3-13)15-5-1-12(9-16(15)25)10-19(29)30/h1,4-6,9,11,13H,2-3,7-8,10H2,(H,27,31)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.23E+3 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188263
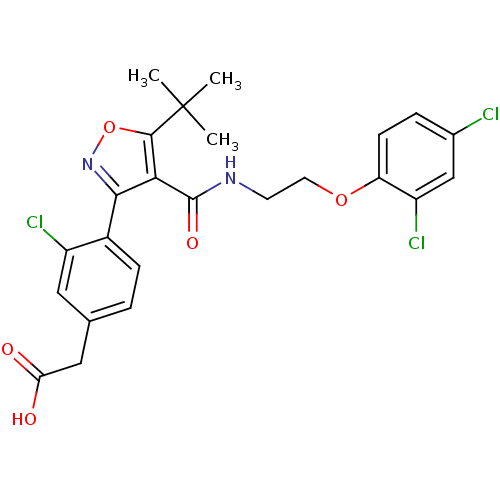
(2-(4-(5-tert-butyl-4-((2-(2,4-dichlorophenoxy)ethy...)Show SMILES CC(C)(C)c1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C24H23Cl3N2O5/c1-24(2,3)22-20(23(32)28-8-9-33-18-7-5-14(25)12-17(18)27)21(29-34-22)15-6-4-13(10-16(15)26)11-19(30)31/h4-7,10,12H,8-9,11H2,1-3H3,(H,28,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28764

(2-[3-chloro-4-(4-{[2-(2,4-dichlorophenoxy)ethyl]ca...)Show SMILES OC(=O)Cc1ccc(-c2noc(c2C(=O)NCCOc2ccc(Cl)cc2Cl)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C26H19Cl3N2O5/c27-17-7-9-21(20(29)14-17)35-11-10-30-26(34)23-24(31-36-25(23)16-4-2-1-3-5-16)18-8-6-15(12-19(18)28)13-22(32)33/h1-9,12,14H,10-11,13H2,(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188264
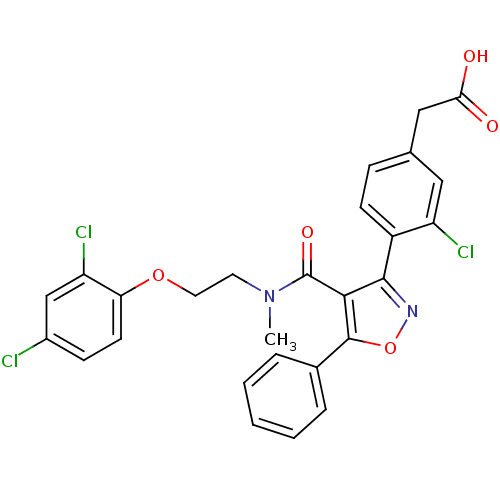
(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)(methyl)car...)Show SMILES CN(CCOc1ccc(Cl)cc1Cl)C(=O)c1c(noc1-c1ccccc1)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C27H21Cl3N2O5/c1-32(11-12-36-22-10-8-18(28)15-21(22)30)27(35)24-25(31-37-26(24)17-5-3-2-4-6-17)19-9-7-16(13-20(19)29)14-23(33)34/h2-10,13,15H,11-12,14H2,1H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188264

(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)(methyl)car...)Show SMILES CN(CCOc1ccc(Cl)cc1Cl)C(=O)c1c(noc1-c1ccccc1)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C27H21Cl3N2O5/c1-32(11-12-36-22-10-8-18(28)15-21(22)30)27(35)24-25(31-37-26(24)17-5-3-2-4-6-17)19-9-7-16(13-20(19)29)14-23(33)34/h2-10,13,15H,11-12,14H2,1H3,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188255

(2-(3-chloro-4-(5-methyl-4-((2-(2-nitrophenoxy)ethy...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccccc1[N+]([O-])=O)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C21H18ClN3O7/c1-12-19(20(24-32-12)14-7-6-13(10-15(14)22)11-18(26)27)21(28)23-8-9-31-17-5-3-2-4-16(17)25(29)30/h2-7,10H,8-9,11H2,1H3,(H,23,28)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188257

(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C21H17Cl3N2O5/c1-11-19(21(29)25-6-7-30-17-5-3-13(22)10-16(17)24)20(26-31-11)14-4-2-12(8-15(14)23)9-18(27)28/h2-5,8,10H,6-7,9H2,1H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28764

(2-[3-chloro-4-(4-{[2-(2,4-dichlorophenoxy)ethyl]ca...)Show SMILES OC(=O)Cc1ccc(-c2noc(c2C(=O)NCCOc2ccc(Cl)cc2Cl)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C26H19Cl3N2O5/c27-17-7-9-21(20(29)14-17)35-11-10-30-26(34)23-24(31-36-25(23)16-4-2-1-3-5-16)18-8-6-15(12-19(18)28)13-22(32)33/h1-9,12,14H,10-11,13H2,(H,30,34)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188252
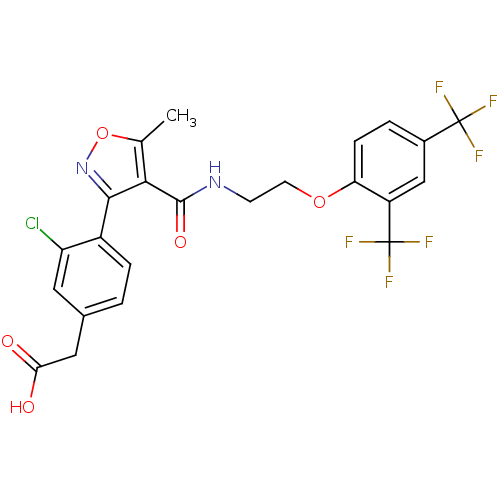
(2-(4-(4-((2-(2,4-bis(trifluoromethyl)phenoxy)ethyl...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(cc1C(F)(F)F)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C23H17ClF6N2O5/c1-11-19(20(32-37-11)14-4-2-12(8-16(14)24)9-18(33)34)21(35)31-6-7-36-17-5-3-13(22(25,26)27)10-15(17)23(28,29)30/h2-5,8,10H,6-7,9H2,1H3,(H,31,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188250

(2-(2-nitro-4-(trifluoromethyl)phenoxy)ethyl 3-(2,6...)Show SMILES Cc1onc(c1C(=O)OCCOc1ccc(cc1[N+]([O-])=O)C(F)(F)F)-c1c(Cl)cccc1Cl |(-2.32,-20.07,;-3.85,-19.91,;-4.63,-18.58,;-6.14,-18.91,;-6.28,-20.44,;-4.88,-21.06,;-4.12,-22.4,;-4.89,-23.73,;-2.58,-22.41,;-1.79,-21.09,;-.25,-21.1,;.53,-19.77,;2.07,-19.79,;2.82,-21.12,;4.36,-21.14,;5.14,-19.81,;4.37,-18.46,;2.84,-18.46,;2.07,-17.12,;2.84,-15.79,;.53,-17.11,;6.68,-19.82,;8.22,-19.82,;6.68,-21.36,;6.69,-18.28,;-7.61,-21.22,;-8.95,-20.45,;-8.95,-18.91,;-10.28,-21.23,;-10.28,-22.77,;-8.94,-23.54,;-7.61,-22.77,;-6.27,-23.54,)| Show InChI InChI=1S/C20H13Cl2F3N2O6/c1-10-16(18(26-33-10)17-12(21)3-2-4-13(17)22)19(28)32-8-7-31-15-6-5-11(20(23,24)25)9-14(15)27(29)30/h2-6,9H,7-8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188261

(2-(3-chloro-4-(5-methyl-4-((2-(4-(trifluoromethyl)...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(cc1)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H18ClF3N2O5/c1-12-19(20(28-33-12)16-7-2-13(10-17(16)23)11-18(29)30)21(31)27-8-9-32-15-5-3-14(4-6-15)22(24,25)26/h2-7,10H,8-9,11H2,1H3,(H,27,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188256

(2-(3-chloro-4-(4-(4-(2,4-dichlorophenoxy)butanoyl)...)Show SMILES OC(=O)Cc1ccc(-c2noc(c2C(=O)CCCOc2ccc(Cl)cc2Cl)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C27H20Cl3NO5/c28-18-9-11-23(21(30)15-18)35-12-4-7-22(32)25-26(31-36-27(25)17-5-2-1-3-6-17)19-10-8-16(13-20(19)29)14-24(33)34/h1-3,5-6,8-11,13,15H,4,7,12,14H2,(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188258

(2-(3-chloro-4-(5-cyclopropyl-4-((2-(2,4-dichloroph...)Show SMILES OC(=O)Cc1ccc(-c2noc(C3CC3)c2C(=O)NCCOc2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C23H19Cl3N2O5/c24-14-4-6-18(17(26)11-14)32-8-7-27-23(31)20-21(28-33-22(20)13-2-3-13)15-5-1-12(9-16(15)25)10-19(29)30/h1,4-6,9,11,13H,2-3,7-8,10H2,(H,27,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188259

(2-(3-chloro-4-(5-methyl-4-((2-(2-nitro-4-(trifluor...)Show SMILES Cc1onc(c1COCCOc1ccc(cc1[N+]([O-])=O)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H18ClF3N2O7/c1-12-16(21(27-35-12)15-4-2-13(8-17(15)23)9-20(29)30)11-33-6-7-34-19-5-3-14(22(24,25)26)10-18(19)28(31)32/h2-5,8,10H,6-7,9,11H2,1H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188256

(2-(3-chloro-4-(4-(4-(2,4-dichlorophenoxy)butanoyl)...)Show SMILES OC(=O)Cc1ccc(-c2noc(c2C(=O)CCCOc2ccc(Cl)cc2Cl)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C27H20Cl3NO5/c28-18-9-11-23(21(30)15-18)35-12-4-7-22(32)25-26(31-36-27(25)17-5-2-1-3-6-17)19-10-8-16(13-20(19)29)14-24(33)34/h1-3,5-6,8-11,13,15H,4,7,12,14H2,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188263

(2-(4-(5-tert-butyl-4-((2-(2,4-dichlorophenoxy)ethy...)Show SMILES CC(C)(C)c1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C24H23Cl3N2O5/c1-24(2,3)22-20(23(32)28-8-9-33-18-7-5-14(25)12-17(18)27)21(29-34-22)15-6-4-13(10-16(15)26)11-19(30)31/h4-7,10,12H,8-9,11H2,1-3H3,(H,28,32)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188260
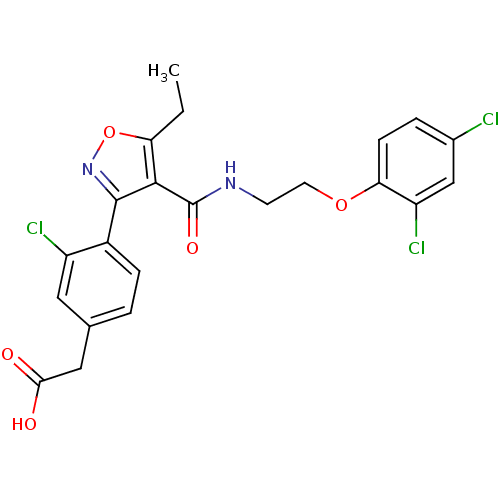
(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES CCc1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H19Cl3N2O5/c1-2-17-20(22(30)26-7-8-31-18-6-4-13(23)11-16(18)25)21(27-32-17)14-5-3-12(9-15(14)24)10-19(28)29/h3-6,9,11H,2,7-8,10H2,1H3,(H,26,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188251
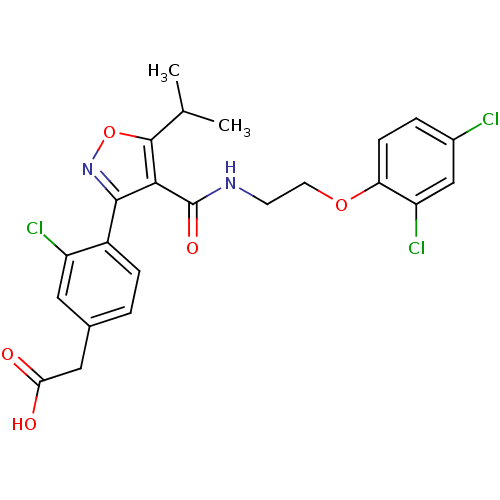
(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES CC(C)c1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C23H21Cl3N2O5/c1-12(2)22-20(23(31)27-7-8-32-18-6-4-14(24)11-17(18)26)21(28-33-22)15-5-3-13(9-16(15)25)10-19(29)30/h3-6,9,11-12H,7-8,10H2,1-2H3,(H,27,31)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188253

(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES OC(=O)Cc1ccc(-c2noc(-c3ccco3)c2C(=O)NCCOc2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C24H17Cl3N2O6/c25-14-4-6-18(17(27)12-14)34-9-7-28-24(32)21-22(29-35-23(21)19-2-1-8-33-19)15-5-3-13(10-16(15)26)11-20(30)31/h1-6,8,10,12H,7,9,11H2,(H,28,32)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188254
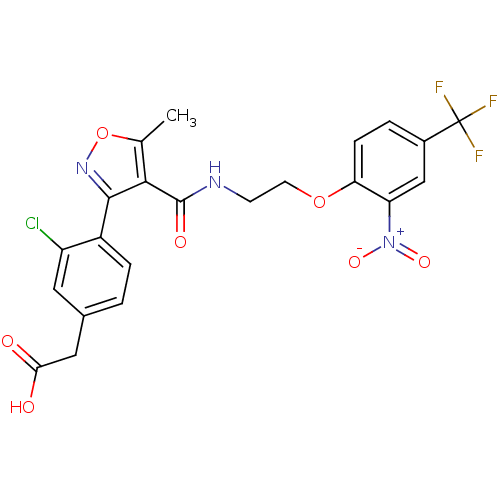
(2-(3-chloro-4-(5-methyl-4-((2-(2-nitro-4-(trifluor...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(cc1[N+]([O-])=O)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H17ClF3N3O7/c1-11-19(20(28-36-11)14-4-2-12(8-15(14)23)9-18(30)31)21(32)27-6-7-35-17-5-3-13(22(24,25)26)10-16(17)29(33)34/h2-5,8,10H,6-7,9H2,1H3,(H,27,32)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188252

(2-(4-(4-((2-(2,4-bis(trifluoromethyl)phenoxy)ethyl...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(cc1C(F)(F)F)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C23H17ClF6N2O5/c1-11-19(20(32-37-11)14-4-2-12(8-16(14)24)9-18(33)34)21(35)31-6-7-36-17-5-3-13(22(25,26)27)10-15(17)23(28,29)30/h2-5,8,10H,6-7,9H2,1H3,(H,31,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188254

(2-(3-chloro-4-(5-methyl-4-((2-(2-nitro-4-(trifluor...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(cc1[N+]([O-])=O)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H17ClF3N3O7/c1-11-19(20(28-36-11)14-4-2-12(8-15(14)23)9-18(30)31)21(32)27-6-7-35-17-5-3-13(22(24,25)26)10-16(17)29(33)34/h2-5,8,10H,6-7,9H2,1H3,(H,27,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188260

(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES CCc1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H19Cl3N2O5/c1-2-17-20(22(30)26-7-8-31-18-6-4-13(23)11-16(18)25)21(27-32-17)14-5-3-12(9-15(14)24)10-19(28)29/h3-6,9,11H,2,7-8,10H2,1H3,(H,26,30)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188261

(2-(3-chloro-4-(5-methyl-4-((2-(4-(trifluoromethyl)...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(cc1)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H18ClF3N2O5/c1-12-19(20(28-33-12)16-7-2-13(10-17(16)23)11-18(29)30)21(31)27-8-9-32-15-5-3-14(4-6-15)22(24,25)26/h2-7,10H,8-9,11H2,1H3,(H,27,31)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188262
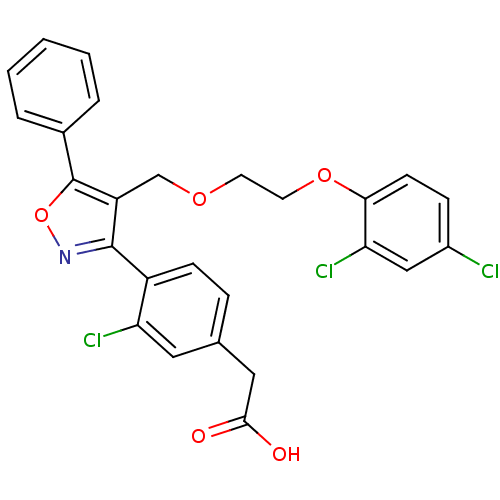
(2-(3-chloro-4-(4-((2-(2,4-dichlorophenoxy)ethoxy)m...)Show SMILES OC(=O)Cc1ccc(-c2noc(c2COCCOc2ccc(Cl)cc2Cl)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C26H20Cl3NO5/c27-18-7-9-23(22(29)14-18)34-11-10-33-15-20-25(30-35-26(20)17-4-2-1-3-5-17)19-8-6-16(12-21(19)28)13-24(31)32/h1-9,12,14H,10-11,13,15H2,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188250

(2-(2-nitro-4-(trifluoromethyl)phenoxy)ethyl 3-(2,6...)Show SMILES Cc1onc(c1C(=O)OCCOc1ccc(cc1[N+]([O-])=O)C(F)(F)F)-c1c(Cl)cccc1Cl |(-2.32,-20.07,;-3.85,-19.91,;-4.63,-18.58,;-6.14,-18.91,;-6.28,-20.44,;-4.88,-21.06,;-4.12,-22.4,;-4.89,-23.73,;-2.58,-22.41,;-1.79,-21.09,;-.25,-21.1,;.53,-19.77,;2.07,-19.79,;2.82,-21.12,;4.36,-21.14,;5.14,-19.81,;4.37,-18.46,;2.84,-18.46,;2.07,-17.12,;2.84,-15.79,;.53,-17.11,;6.68,-19.82,;8.22,-19.82,;6.68,-21.36,;6.69,-18.28,;-7.61,-21.22,;-8.95,-20.45,;-8.95,-18.91,;-10.28,-21.23,;-10.28,-22.77,;-8.94,-23.54,;-7.61,-22.77,;-6.27,-23.54,)| Show InChI InChI=1S/C20H13Cl2F3N2O6/c1-10-16(18(26-33-10)17-12(21)3-2-4-13(17)22)19(28)32-8-7-31-15-6-5-11(20(23,24)25)9-14(15)27(29)30/h2-6,9H,7-8H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188252

(2-(4-(4-((2-(2,4-bis(trifluoromethyl)phenoxy)ethyl...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(cc1C(F)(F)F)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C23H17ClF6N2O5/c1-11-19(20(32-37-11)14-4-2-12(8-16(14)24)9-18(33)34)21(35)31-6-7-36-17-5-3-13(22(25,26)27)10-15(17)23(28,29)30/h2-5,8,10H,6-7,9H2,1H3,(H,31,35)(H,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188255

(2-(3-chloro-4-(5-methyl-4-((2-(2-nitrophenoxy)ethy...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccccc1[N+]([O-])=O)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C21H18ClN3O7/c1-12-19(20(24-32-12)14-7-6-13(10-15(14)22)11-18(26)27)21(28)23-8-9-31-17-5-3-2-4-16(17)25(29)30/h2-7,10H,8-9,11H2,1H3,(H,23,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188251

(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES CC(C)c1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C23H21Cl3N2O5/c1-12(2)22-20(23(31)27-7-8-32-18-6-4-14(24)11-17(18)26)21(28-33-22)15-5-3-13(9-16(15)25)10-19(29)30/h3-6,9,11-12H,7-8,10H2,1-2H3,(H,27,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188254

(2-(3-chloro-4-(5-methyl-4-((2-(2-nitro-4-(trifluor...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(cc1[N+]([O-])=O)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H17ClF3N3O7/c1-11-19(20(28-36-11)14-4-2-12(8-15(14)23)9-18(30)31)21(32)27-6-7-35-17-5-3-13(22(24,25)26)10-16(17)29(33)34/h2-5,8,10H,6-7,9H2,1H3,(H,27,32)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28661

(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM28661

(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188259

(2-(3-chloro-4-(5-methyl-4-((2-(2-nitro-4-(trifluor...)Show SMILES Cc1onc(c1COCCOc1ccc(cc1[N+]([O-])=O)C(F)(F)F)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H18ClF3N2O7/c1-12-16(21(27-35-12)15-4-2-13(8-17(15)23)9-20(29)30)11-33-6-7-34-19-5-3-14(22(24,25)26)10-18(19)28(31)32/h2-5,8,10H,6-7,9,11H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188253

(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES OC(=O)Cc1ccc(-c2noc(-c3ccco3)c2C(=O)NCCOc2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C24H17Cl3N2O6/c25-14-4-6-18(17(27)12-14)34-9-7-28-24(32)21-22(29-35-23(21)19-2-1-8-33-19)15-5-3-13(10-16(15)26)11-20(30)31/h1-6,8,10,12H,7,9,11H2,(H,28,32)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188262

(2-(3-chloro-4-(4-((2-(2,4-dichlorophenoxy)ethoxy)m...)Show SMILES OC(=O)Cc1ccc(-c2noc(c2COCCOc2ccc(Cl)cc2Cl)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C26H20Cl3NO5/c27-18-7-9-23(22(29)14-18)34-11-10-33-15-20-25(30-35-26(20)17-4-2-1-3-5-17)19-8-6-16(12-21(19)28)13-24(31)32/h1-9,12,14H,10-11,13,15H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188257

(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES Cc1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C21H17Cl3N2O5/c1-11-19(21(29)25-6-7-30-17-5-3-13(22)10-16(17)24)20(26-31-11)14-4-2-12(8-15(14)23)9-18(27)28/h2-5,8,10H,6-7,9H2,1H3,(H,25,29)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50188264

(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)(methyl)car...)Show SMILES CN(CCOc1ccc(Cl)cc1Cl)C(=O)c1c(noc1-c1ccccc1)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C27H21Cl3N2O5/c1-32(11-12-36-22-10-8-18(28)15-21(22)30)27(35)24-25(31-37-26(24)17-5-3-2-4-6-17)19-9-7-16(13-20(19)29)14-23(33)34/h2-10,13,15H,11-12,14H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARgamma by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188250

(2-(2-nitro-4-(trifluoromethyl)phenoxy)ethyl 3-(2,6...)Show SMILES Cc1onc(c1C(=O)OCCOc1ccc(cc1[N+]([O-])=O)C(F)(F)F)-c1c(Cl)cccc1Cl |(-2.32,-20.07,;-3.85,-19.91,;-4.63,-18.58,;-6.14,-18.91,;-6.28,-20.44,;-4.88,-21.06,;-4.12,-22.4,;-4.89,-23.73,;-2.58,-22.41,;-1.79,-21.09,;-.25,-21.1,;.53,-19.77,;2.07,-19.79,;2.82,-21.12,;4.36,-21.14,;5.14,-19.81,;4.37,-18.46,;2.84,-18.46,;2.07,-17.12,;2.84,-15.79,;.53,-17.11,;6.68,-19.82,;8.22,-19.82,;6.68,-21.36,;6.69,-18.28,;-7.61,-21.22,;-8.95,-20.45,;-8.95,-18.91,;-10.28,-21.23,;-10.28,-22.77,;-8.94,-23.54,;-7.61,-22.77,;-6.27,-23.54,)| Show InChI InChI=1S/C20H13Cl2F3N2O6/c1-10-16(18(26-33-10)17-12(21)3-2-4-13(17)22)19(28)32-8-7-31-15-6-5-11(20(23,24)25)9-14(15)27(29)30/h2-6,9H,7-8H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188262

(2-(3-chloro-4-(4-((2-(2,4-dichlorophenoxy)ethoxy)m...)Show SMILES OC(=O)Cc1ccc(-c2noc(c2COCCOc2ccc(Cl)cc2Cl)-c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C26H20Cl3NO5/c27-18-7-9-23(22(29)14-18)34-11-10-33-15-20-25(30-35-26(20)17-4-2-1-3-5-17)19-8-6-16(12-21(19)28)13-24(31)32/h1-9,12,14H,10-11,13,15H2,(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188260

(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES CCc1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C22H19Cl3N2O5/c1-2-17-20(22(30)26-7-8-31-18-6-4-13(23)11-16(18)25)21(27-32-17)14-5-3-12(9-15(14)24)10-19(28)29/h3-6,9,11H,2,7-8,10H2,1H3,(H,26,30)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50188251

(2-(4-(4-((2-(2,4-dichlorophenoxy)ethyl)carbamoyl)-...)Show SMILES CC(C)c1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C23H21Cl3N2O5/c1-12(2)22-20(23(31)27-7-8-32-18-6-4-14(24)11-17(18)26)21(28-33-22)15-5-3-13(9-16(15)25)10-19(29)30/h3-6,9,11-12H,7-8,10H2,1-2H3,(H,27,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.11E+3 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARalpha by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188263

(2-(4-(5-tert-butyl-4-((2-(2,4-dichlorophenoxy)ethy...)Show SMILES CC(C)(C)c1onc(c1C(=O)NCCOc1ccc(Cl)cc1Cl)-c1ccc(CC(O)=O)cc1Cl Show InChI InChI=1S/C24H23Cl3N2O5/c1-24(2,3)22-20(23(32)28-8-9-33-18-7-5-14(25)12-17(18)27)21(29-34-22)15-6-4-13(10-16(15)26)11-19(30)31/h4-7,10,12H,8-9,11H2,1-3H3,(H,28,32)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.59E+3 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50188258

(2-(3-chloro-4-(5-cyclopropyl-4-((2-(2,4-dichloroph...)Show SMILES OC(=O)Cc1ccc(-c2noc(C3CC3)c2C(=O)NCCOc2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C23H19Cl3N2O5/c24-14-4-6-18(17(26)11-14)32-8-7-27-23(31)20-21(28-33-22(20)13-2-3-13)15-5-1-12(9-16(15)25)10-19(29)30/h1,4-6,9,11,13H,2-3,7-8,10H2,(H,27,31)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at PPARdelta by transactivation assay |
Bioorg Med Chem Lett 16: 4376-80 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.055
BindingDB Entry DOI: 10.7270/Q2V987PC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data