

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188534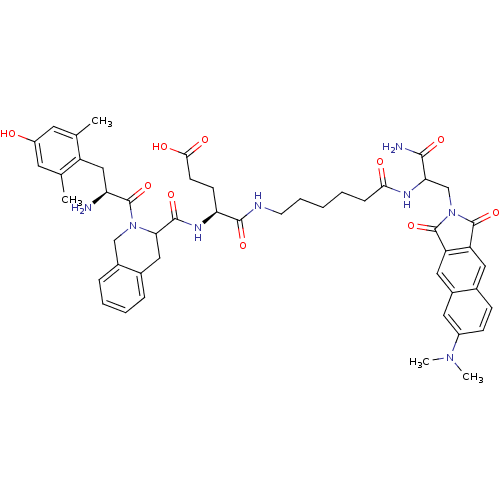 ((H-Dmt-Tic-Glu-NH-(CH(2))(5)-CO-Dap(6DMN)-NH(2) | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin C from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188537 (CHEMBL208916 | H-Dmt-Tic-Glu-Dap(6DMN)-NH(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin C from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188534 ((H-Dmt-Tic-Glu-NH-(CH(2))(5)-CO-Dap(6DMN)-NH(2) | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188533 (CHEMBL411335 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-(C=O)-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188535 (CHEMBL219407 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-NH-(C=S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188535 (CHEMBL219407 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-NH-(C=S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 668 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin C from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188536 (CHEMBL207661 | H-Tyr-Pro-Dap(6DMN)-Phe-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 839 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin C from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188536 (CHEMBL207661 | H-Tyr-Pro-Dap(6DMN)-Phe-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188533 (CHEMBL411335 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-(C=O)-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]deltorphin C from rat delta opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50188537 (CHEMBL208916 | H-Dmt-Tic-Glu-Dap(6DMN)-NH(2)) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat mu opioid receptor in brain P2 synaptosomes | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50188537 (CHEMBL208916 | H-Dmt-Tic-Glu-Dap(6DMN)-NH(2)) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against mu opioid receptor in guinea-pig ileum | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50188535 (CHEMBL219407 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-NH-(C=S...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against mu opioid receptor in guinea-pig ileum | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50188535 (CHEMBL219407 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-NH-(C=S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against delta opioid receptor in mouse vas deferens | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50188534 ((H-Dmt-Tic-Glu-NH-(CH(2))(5)-CO-Dap(6DMN)-NH(2) | ...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against mu opioid receptor in guinea-pig ileum | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50188533 (CHEMBL411335 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-(C=O)-D...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against mu opioid receptor in guinea-pig ileum | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50188533 (CHEMBL411335 | H-Tyr-Pro-Phe-Phe-NH-(CH2)5-(C=O)-D...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against delta opioid receptor in mouse vas deferens | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50188536 (CHEMBL207661 | H-Tyr-Pro-Dap(6DMN)-Phe-NH2) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against mu opioid receptor in guinea-pig ileum | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50188536 (CHEMBL207661 | H-Tyr-Pro-Dap(6DMN)-Phe-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description Functional bioactivity against delta opioid receptor in mouse vas deferens | J Med Chem 49: 3653-8 (2006) Article DOI: 10.1021/jm060343t BindingDB Entry DOI: 10.7270/Q25M65B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||