 Found 85 hits of Enzyme Inhibition Constant Data
Found 85 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192076

(1-{5-[((S)-5-chloro-indan-2-ylamino)-methyl]-1H-py...)Show SMILES Clc1ccc2C[C@@H](Cc2c1)NCc1cc(NC(=O)Nc2cccc3C(=O)N4CCC[C@H]4c23)[nH]n1 Show InChI InChI=1S/C25H25ClN6O2/c26-16-7-6-14-10-17(11-15(14)9-16)27-13-18-12-22(31-30-18)29-25(34)28-20-4-1-3-19-23(20)21-5-2-8-32(21)24(19)33/h1,3-4,6-7,9,12,17,21,27H,2,5,8,10-11,13H2,(H3,28,29,30,31,34)/t17-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192074
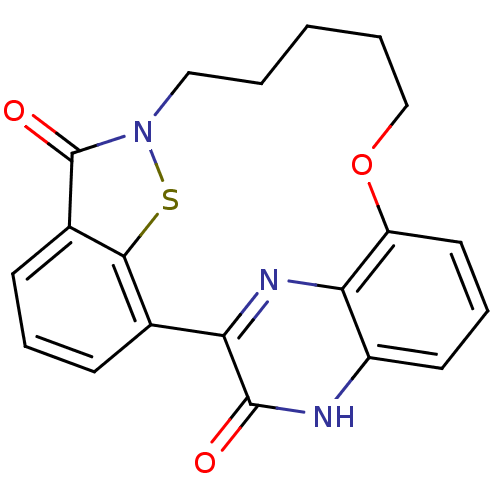
(22-hydroxy-15-oxa-8-thia-9,21,23-triazapentacyclo[...)Show SMILES O=c1n2CCCCCOc3cccc4[nH]c(=O)c(nc34)-c3cccc1c3s2 Show InChI InChI=1S/C20H17N3O3S/c24-19-16-12-6-4-7-13-18(12)27-23(20(13)25)10-2-1-3-11-26-15-9-5-8-14(21-19)17(15)22-16/h4-9H,1-3,10-11H2,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192075
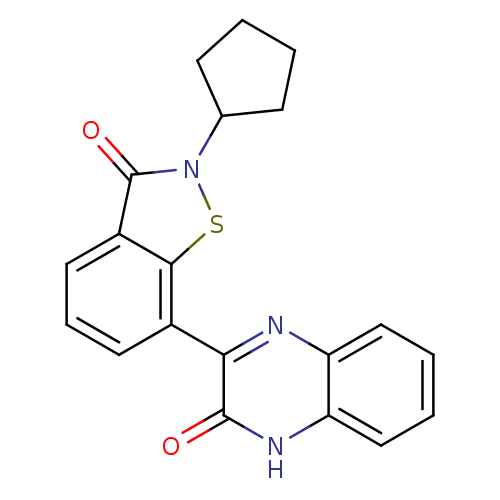
(3-(2-cyclopentyl-3-oxo-2,3-dihydro-benzo[d]isothia...)Show SMILES O=c1n(sc2c(cccc12)-c1nc2ccccc2[nH]c1=O)C1CCCC1 Show InChI InChI=1S/C20H17N3O2S/c24-19-17(21-15-10-3-4-11-16(15)22-19)13-8-5-9-14-18(13)26-23(20(14)25)12-6-1-2-7-12/h3-5,8-12H,1-2,6-7H2,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
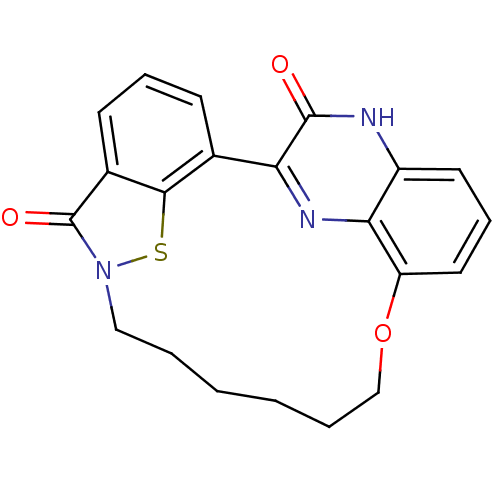
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192073
(22-hydroxy-15-oxa-8-thia-9,12,21,23-tetraazapentac...)Show SMILES O=c1n2CCNCCOc3cccc4[nH]c(=O)c(nc34)-c3cccc1c3s2 Show InChI InChI=1S/C19H16N4O3S/c24-18-15-11-3-1-4-12-17(11)27-23(19(12)25)9-7-20-8-10-26-14-6-2-5-13(21-18)16(14)22-15/h1-6,20H,7-10H2,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192077
(23-hydroxy-16-oxa-8-thia-9,22,24-triazapentacyclo[...)Show SMILES O=c1n2CCCCCCOc3cccc4[nH]c(=O)c(nc34)-c3cccc1c3s2 Show InChI InChI=1S/C21H19N3O3S/c25-20-17-13-7-5-8-14-19(13)28-24(21(14)26)11-3-1-2-4-12-27-16-10-6-9-15(22-20)18(16)23-17/h5-10H,1-4,11-12H2,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192082
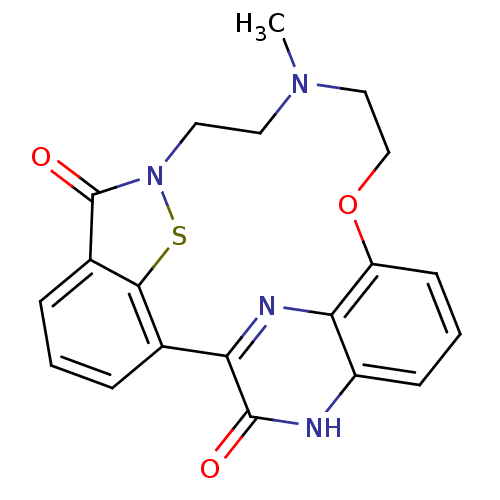
(22-hydroxy-12-methyl-15-oxa-8-thia-9,12,21,23-tetr...)Show SMILES CN1CCOc2cccc3[nH]c(=O)c(nc23)-c2cccc3c2sn(CC1)c3=O Show InChI InChI=1S/C20H18N4O3S/c1-23-8-9-24-20(26)13-5-2-4-12(18(13)28-24)16-19(25)21-14-6-3-7-15(17(14)22-16)27-11-10-23/h2-7H,8-11H2,1H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK6 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192080
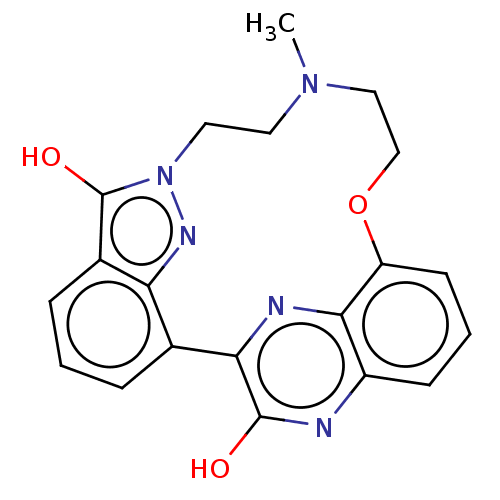
(9-methyl-8,9,10,11-tetrahydro-7H-16,18-(epiminomet...)Show SMILES CN1CCOc2cccc3nc(O)c(nc23)-c2cccc3c(O)n(CC1)nc23 Show InChI InChI=1S/C20H19N5O3/c1-24-8-9-25-20(27)13-5-2-4-12(16(13)23-25)17-19(26)21-14-6-3-7-15(18(14)22-17)28-11-10-24/h2-7,23H,8-11H2,1H3,(H,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192072
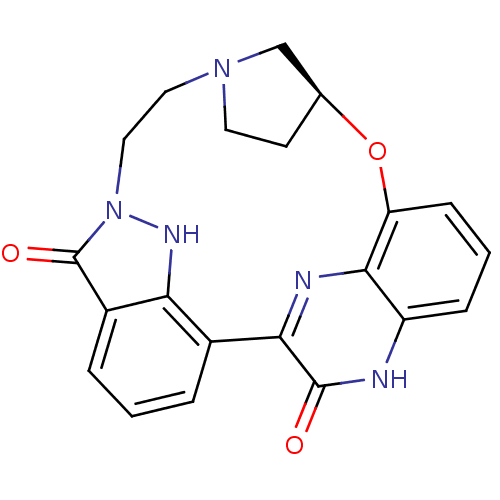
((15S)-16-oxa-8,9,12,22,24-pentaazahexacyclo[15.6.2...)Show SMILES O=c1n2CCN3CC[C@@H](C3)Oc3cccc4[nH]c(=O)c(nc34)-c3cccc1c3[nH]2 Show InChI InChI=1S/C21H19N5O3/c27-20-18-13-3-1-4-14-17(13)24-26(21(14)28)10-9-25-8-7-12(11-25)29-16-6-2-5-15(22-20)19(16)23-18/h1-6,12,24H,7-11H2,(H,22,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM6619
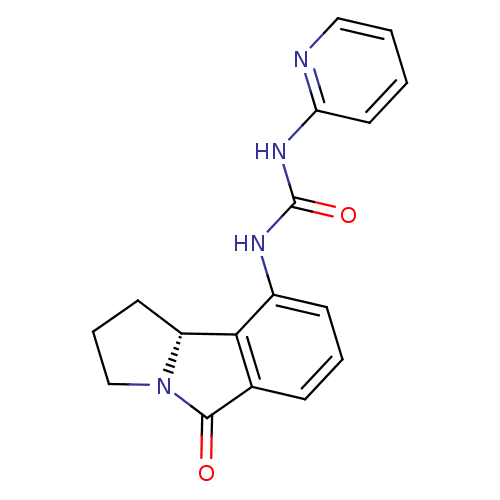
(3-[(9bR)-5-oxo-1H,2H,3H,5H,9bH-benzo[a]pyrrolizin-...)Show SMILES O=C(Nc1ccccn1)Nc1cccc2C(=O)N3CCC[C@@H]3c12 |r| Show InChI InChI=1S/C17H16N4O2/c22-16-11-5-3-6-12(15(11)13-7-4-10-21(13)16)19-17(23)20-14-8-1-2-9-18-14/h1-3,5-6,8-9,13H,4,7,10H2,(H2,18,19,20,23)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6
(Homo sapiens (Human)) | BDBM6619

(3-[(9bR)-5-oxo-1H,2H,3H,5H,9bH-benzo[a]pyrrolizin-...)Show SMILES O=C(Nc1ccccn1)Nc1cccc2C(=O)N3CCC[C@@H]3c12 |r| Show InChI InChI=1S/C17H16N4O2/c22-16-11-5-3-6-12(15(11)13-7-4-10-21(13)16)19-17(23)20-14-8-1-2-9-18-14/h1-3,5-6,8-9,13H,4,7,10H2,(H2,18,19,20,23)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK6 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM6619

(3-[(9bR)-5-oxo-1H,2H,3H,5H,9bH-benzo[a]pyrrolizin-...)Show SMILES O=C(Nc1ccccn1)Nc1cccc2C(=O)N3CCC[C@@H]3c12 |r| Show InChI InChI=1S/C17H16N4O2/c22-16-11-5-3-6-12(15(11)13-7-4-10-21(13)16)19-17(23)20-14-8-1-2-9-18-14/h1-3,5-6,8-9,13H,4,7,10H2,(H2,18,19,20,23)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM6619

(3-[(9bR)-5-oxo-1H,2H,3H,5H,9bH-benzo[a]pyrrolizin-...)Show SMILES O=C(Nc1ccccn1)Nc1cccc2C(=O)N3CCC[C@@H]3c12 |r| Show InChI InChI=1S/C17H16N4O2/c22-16-11-5-3-6-12(15(11)13-7-4-10-21(13)16)19-17(23)20-14-8-1-2-9-18-14/h1-3,5-6,8-9,13H,4,7,10H2,(H2,18,19,20,23)/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192083

(21-hydroxy-14-oxa-8-thia-9,20,22-triazapentacyclo[...)Show SMILES O=c1n2CCCCOc3cccc4[nH]c(=O)c(nc34)-c3cccc1c3s2 Show InChI InChI=1S/C19H15N3O3S/c23-18-15-11-5-3-6-12-17(11)26-22(19(12)24)9-1-2-10-25-14-8-4-7-13(20-18)16(14)21-15/h3-8H,1-2,9-10H2,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192081
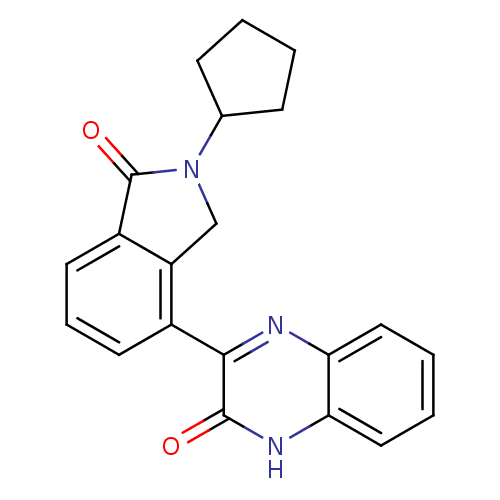
(3-(2-cyclopentyl-1-oxo-2,3-dihydro-1H-isoindol-4-y...)Show SMILES O=C1N(Cc2c1cccc2-c1nc2ccccc2[nH]c1=O)C1CCCC1 Show InChI InChI=1S/C21H19N3O2/c25-20-19(22-17-10-3-4-11-18(17)23-20)14-8-5-9-15-16(14)12-24(21(15)26)13-6-1-2-7-13/h3-5,8-11,13H,1-2,6-7,12H2,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50192076

(1-{5-[((S)-5-chloro-indan-2-ylamino)-methyl]-1H-py...)Show SMILES Clc1ccc2C[C@@H](Cc2c1)NCc1cc(NC(=O)Nc2cccc3C(=O)N4CCC[C@H]4c23)[nH]n1 Show InChI InChI=1S/C25H25ClN6O2/c26-16-7-6-14-10-17(11-15(14)9-16)27-13-18-12-22(31-30-18)29-25(34)28-20-4-1-3-19-23(20)21-5-2-8-32(21)24(19)33/h1,3-4,6-7,9,12,17,21,27H,2,5,8,10-11,13H2,(H3,28,29,30,31,34)/t17-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192079
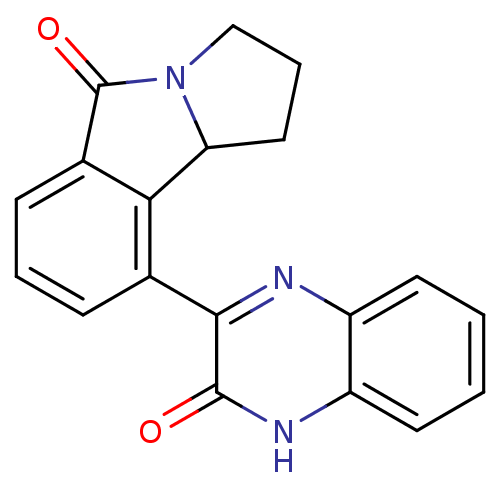
(9-(3-oxo-3,4-dihydro-quinoxalin-2-yl)-1,2,3,9b-tet...)Show InChI InChI=1S/C19H15N3O2/c23-18-17(20-13-7-1-2-8-14(13)21-18)11-5-3-6-12-16(11)15-9-4-10-22(15)19(12)24/h1-3,5-8,15H,4,9-10H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase N2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PRK2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCeta |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Protein kinase C iota type
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCiota |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ERK1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LYN |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1/beta-2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of p706SK |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKBbeta |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SAPK2beta |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Aurora-A |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKBalpha |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Arg kinase |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of RSK2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FLT1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FGFR2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JNK2alpha2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKA |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SAPK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCbeta2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Abl kinase |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type IV
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CamK IV |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SAPK2alpha |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 5
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PRAK |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-RAF |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MAPKAPK2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKBgamma |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JNK1alpha1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Axl kinase |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCmu |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IKK-beta |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Bmx kinase |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Syk |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Blk kinase |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase CSK
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CSK |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IKKalpha |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SGK |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Tie2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MAPK1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Chk1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 6
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MKK6 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 12
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SAPK3 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCepsilon |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ZAP70 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 7
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MKK7beta |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCgamma |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 2
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PAK2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MAPK2 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 4
(Homo sapiens (Human)) | BDBM50192071

((13R,15S)-13-methyl-16-oxa-8,9,12,22,24-pentaazahe...)Show SMILES C[C@@H]1C[C@H]2CN1CCn1[nH]c3c(cccc3c1=O)-c1nc3c(O2)cccc3[nH]c1=O Show InChI InChI=1S/C22H21N5O3/c1-12-10-13-11-26(12)8-9-27-22(29)15-5-2-4-14(18(15)25-27)19-21(28)23-16-6-3-7-17(30-13)20(16)24-19/h2-7,12-13,25H,8-11H2,1H3,(H,23,28)/t12-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MKK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50192076

(1-{5-[((S)-5-chloro-indan-2-ylamino)-methyl]-1H-py...)Show SMILES Clc1ccc2C[C@@H](Cc2c1)NCc1cc(NC(=O)Nc2cccc3C(=O)N4CCC[C@H]4c23)[nH]n1 Show InChI InChI=1S/C25H25ClN6O2/c26-16-7-6-14-10-17(11-15(14)9-16)27-13-18-12-22(31-30-18)29-25(34)28-20-4-1-3-19-23(20)21-5-2-8-32(21)24(19)33/h1,3-4,6-7,9,12,17,21,27H,2,5,8,10-11,13H2,(H3,28,29,30,31,34)/t17-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50192078
(9-(2-oxo-1,4-dihydro-2H-quinazolin-3-yl)-1,2,3,9b-...)Show InChI InChI=1S/C19H17N3O2/c23-18-13-6-3-8-16(17(13)15-9-4-10-21(15)18)22-11-12-5-1-2-7-14(12)20-19(22)24/h1-3,5-8,15H,4,9-11H2,(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 |
Bioorg Med Chem Lett 16: 5122-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.026
BindingDB Entry DOI: 10.7270/Q2K35VF0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data