

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194351 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194342 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194331 (2-hydroxy-2-(5-(2,2,2-trifluoroacetamido)-6-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194352 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194350 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194337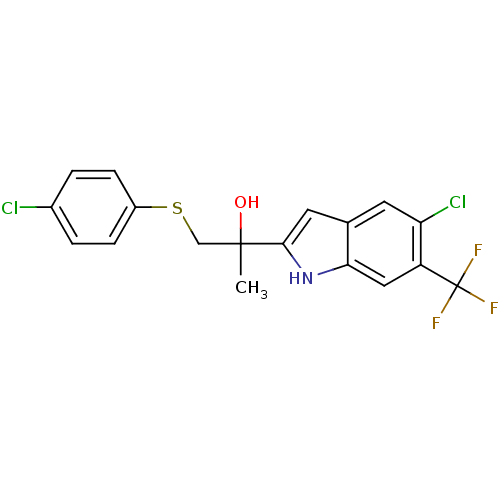 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194330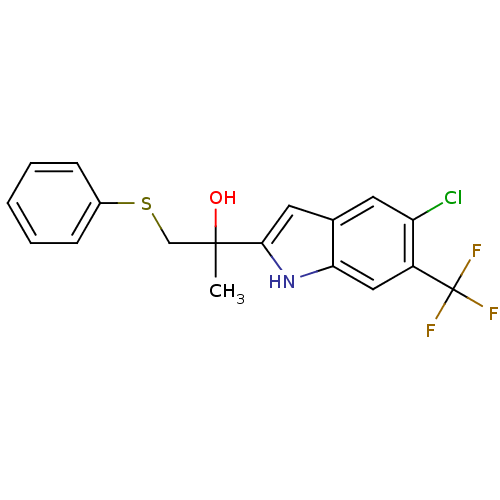 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194353 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-2-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194326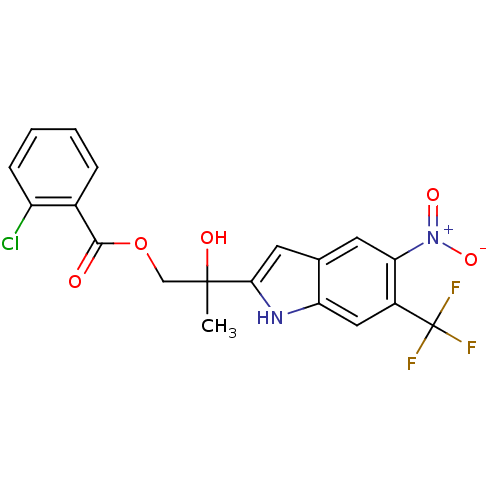 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194340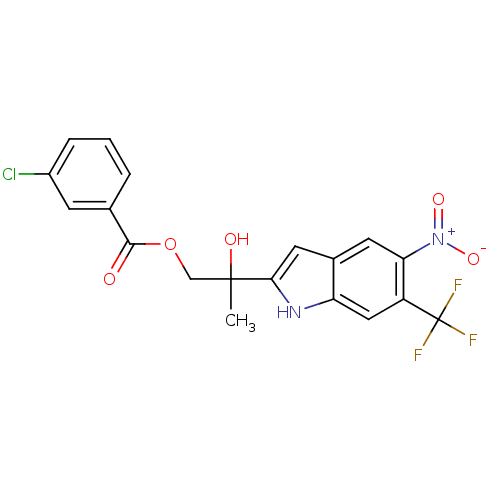 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194345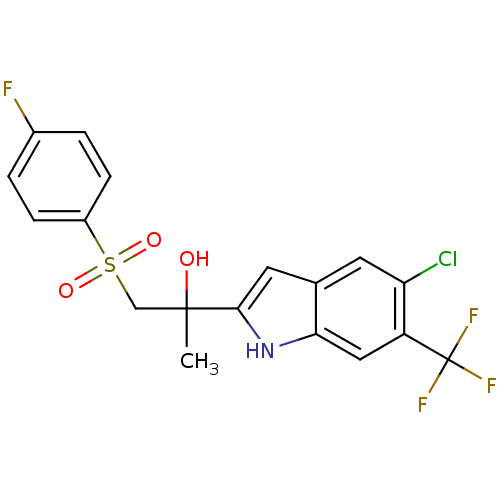 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194327 (2-(5-cyano-6-(trifluoromethyl)-1H-indol-2-yl)-2-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194354 (2-(5-chloro-1H-indol-2-yl)-1-(4-chlorophenylthio)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194344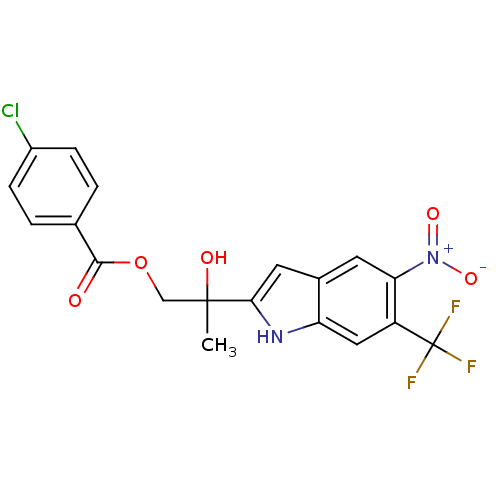 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194346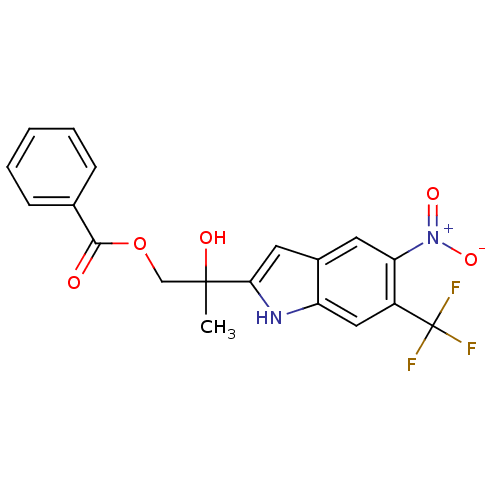 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194328 (1-(4-aminophenylthio)-2-(5-chloro-6-(trifluorometh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194339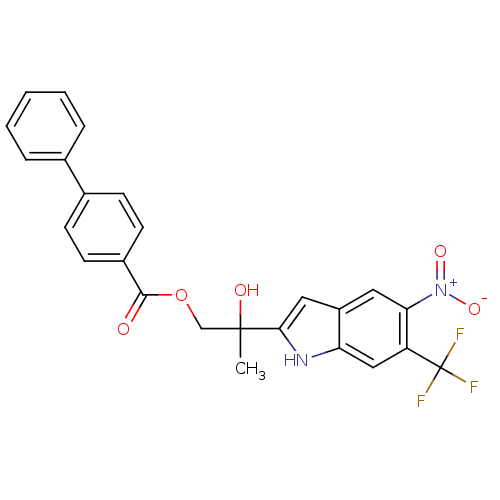 (CHEMBL376323 | biphenyl-4-carboxylic acid 2-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194335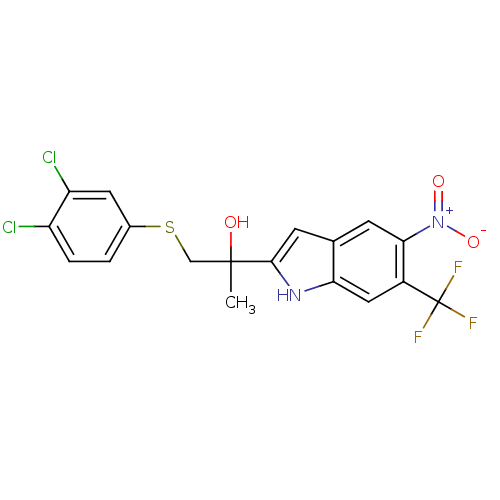 (1-(3,4-dichlorophenylthio)-2-(5-nitro-6-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194355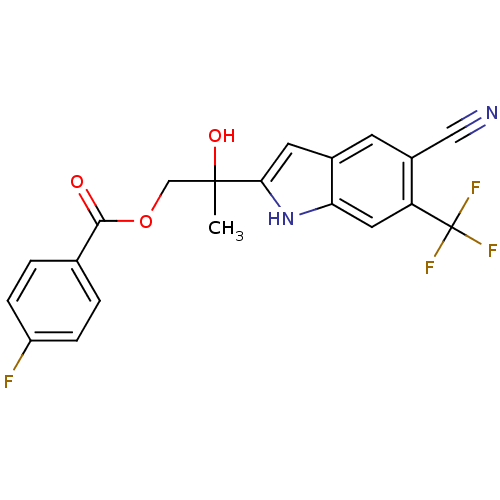 (2-(5-cyano-6-(trifluoromethyl)-1H-indol-2-yl)-2-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM18525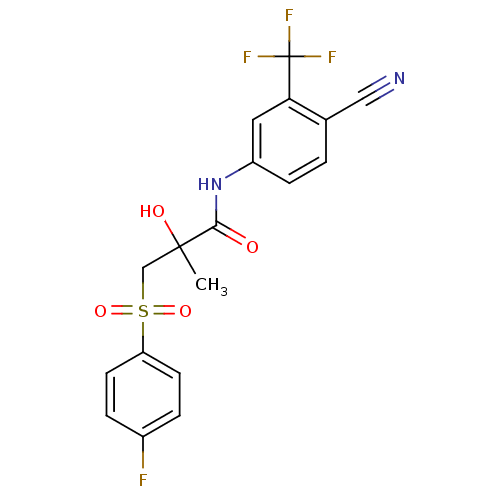 (Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194348 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194347 (2-(1-(4-chlorophenylthio)-2-hydroxypropan-2-yl)-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194332 (2-(1-(4-fluorophenylthio)-2-hydroxypropan-2-yl)-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194349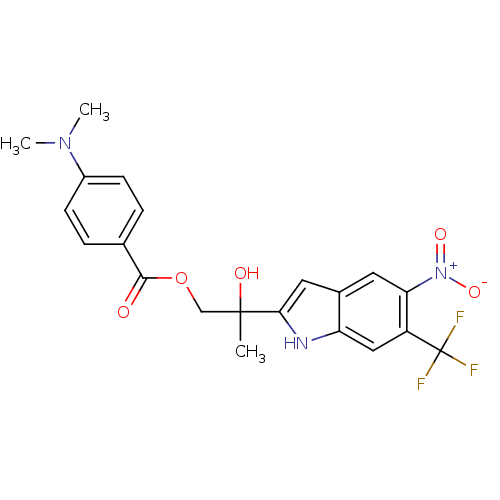 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194333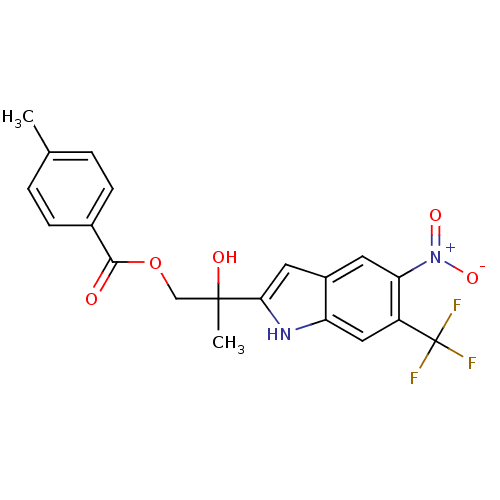 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194341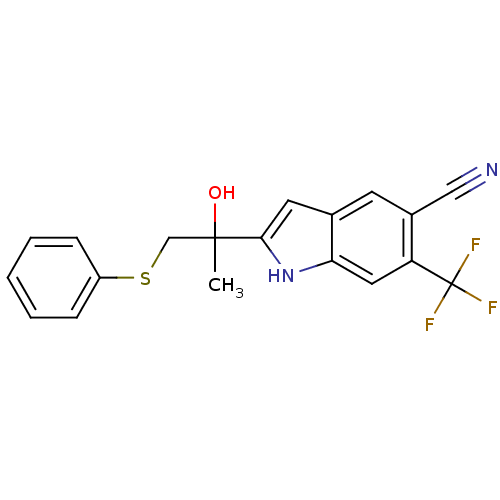 (2-(2-hydroxy-1-(phenylthio)propan-2-yl)-6-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194329 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194338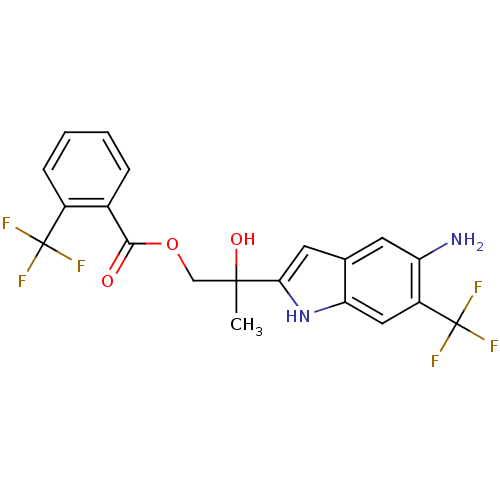 (2-(5-amino-6-(trifluoromethyl)-1H-indol-2-yl)-2-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194336 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194336 (2-(5-chloro-6-(trifluoromethyl)-1H-indol-2-yl)-1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194334 (2-hydroxy-2-(5-nitro-6-(trifluoromethyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM50194343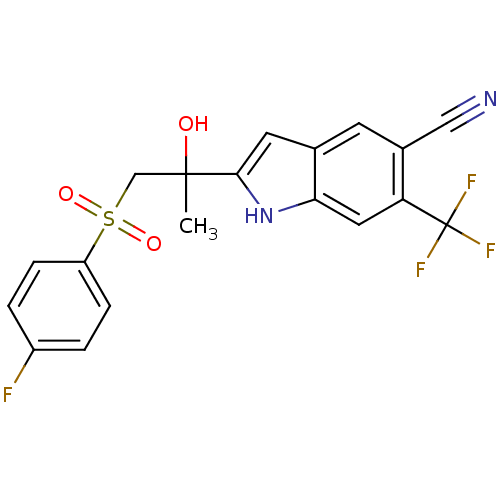 (2-(1-(4-fluorophenylsulfonyl)-2-hydroxypropan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Curated by ChEMBL | Assay Description Displacement of [3H]R1881 from rat androgen receptor | Bioorg Med Chem Lett 16: 5646-9 (2006) Article DOI: 10.1016/j.bmcl.2006.08.036 BindingDB Entry DOI: 10.7270/Q24Q7VSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||