 Found 24 hits of Enzyme Inhibition Constant Data
Found 24 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194364
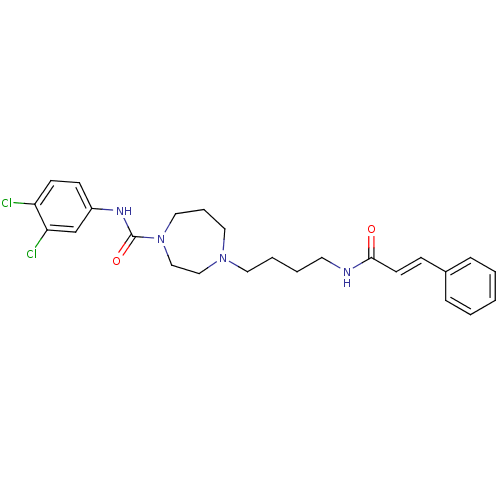
(4-(4-cinnamamidobutyl)-N-(3,4-dichlorophenyl)-1,4-...)Show SMILES Clc1ccc(NC(=O)N2CCCN(CCCCNC(=O)\C=C\c3ccccc3)CC2)cc1Cl Show InChI InChI=1S/C25H30Cl2N4O2/c26-22-11-10-21(19-23(22)27)29-25(33)31-16-6-15-30(17-18-31)14-5-4-13-28-24(32)12-9-20-7-2-1-3-8-20/h1-3,7-12,19H,4-6,13-18H2,(H,28,32)(H,29,33)/b12-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194380
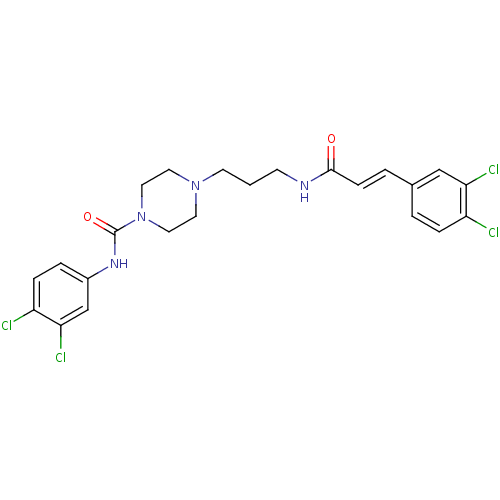
(CHEMBL223087 | N-(3,4-dichlorophenyl)-4-(3-(3-(3,4...)Show SMILES Clc1ccc(NC(=O)N2CCN(CCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C23H24Cl4N4O2/c24-18-5-2-16(14-20(18)26)3-7-22(32)28-8-1-9-30-10-12-31(13-11-30)23(33)29-17-4-6-19(25)21(27)15-17/h2-7,14-15H,1,8-13H2,(H,28,32)(H,29,33)/b7-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194365

(CHEMBL219730 | N-(3,4-dichlorophenyl)-4-(6-(3-(3,4...)Show SMILES Clc1ccc(NC(=O)N2CCN(CCCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C26H30Cl4N4O2/c27-21-8-5-19(17-23(21)29)6-10-25(35)31-11-3-1-2-4-12-33-13-15-34(16-14-33)26(36)32-20-7-9-22(28)24(30)18-20/h5-10,17-18H,1-4,11-16H2,(H,31,35)(H,32,36)/b10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194374
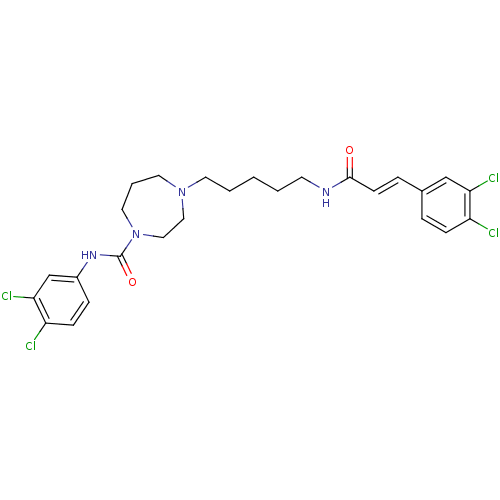
(CHEMBL375397 | N-(3,4-dichlorophenyl)-4-(5-(3-(3,4...)Show SMILES Clc1ccc(NC(=O)N2CCCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C26H30Cl4N4O2/c27-21-8-5-19(17-23(21)29)6-10-25(35)31-11-2-1-3-12-33-13-4-14-34(16-15-33)26(36)32-20-7-9-22(28)24(30)18-20/h5-10,17-18H,1-4,11-16H2,(H,31,35)(H,32,36)/b10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194375
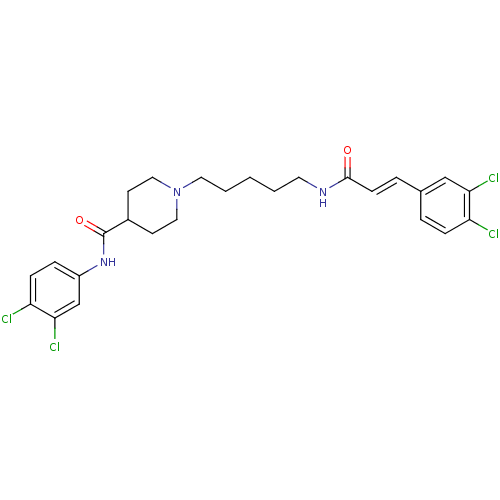
(CHEMBL373869 | N-(3,4-dichlorophenyl)-1-(5-(3-(3,4...)Show SMILES Clc1ccc(NC(=O)C2CCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C26H29Cl4N3O2/c27-21-7-4-18(16-23(21)29)5-9-25(34)31-12-2-1-3-13-33-14-10-19(11-15-33)26(35)32-20-6-8-22(28)24(30)17-20/h4-9,16-17,19H,1-3,10-15H2,(H,31,34)(H,32,35)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194376
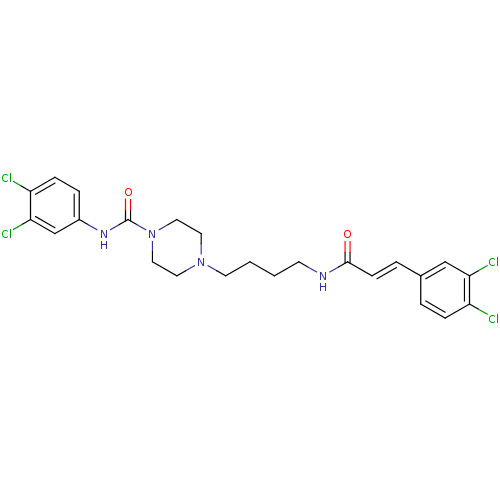
(CHEMBL221226 | N-(3,4-dichlorophenyl)-4-(4-(3-(3,4...)Show SMILES Clc1ccc(NC(=O)N2CCN(CCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C24H26Cl4N4O2/c25-19-6-3-17(15-21(19)27)4-8-23(33)29-9-1-2-10-31-11-13-32(14-12-31)24(34)30-18-5-7-20(26)22(28)16-18/h3-8,15-16H,1-2,9-14H2,(H,29,33)(H,30,34)/b8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194370

(CHEMBL220954 | N-(3-chloro-4-fluorophenyl)-4-(5-(3...)Show SMILES Fc1ccc(NC(=O)N2CCCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C26H30Cl3FN4O2/c27-21-8-5-19(17-22(21)28)6-10-25(35)31-11-2-1-3-12-33-13-4-14-34(16-15-33)26(36)32-20-7-9-24(30)23(29)18-20/h5-10,17-18H,1-4,11-16H2,(H,31,35)(H,32,36)/b10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194363
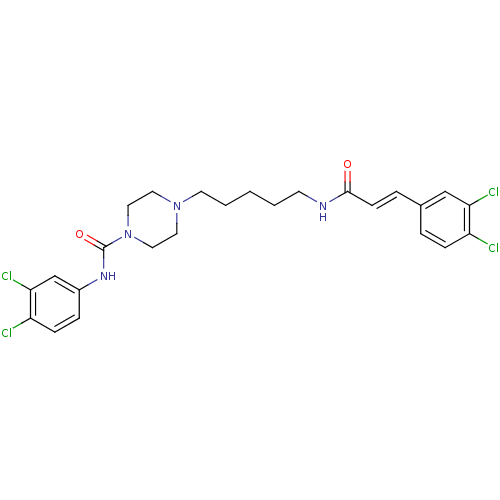
(CHEMBL221442 | N-(3,4-dichlorophenyl)-4-(5-(3-(3,4...)Show SMILES Clc1ccc(NC(=O)N2CCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C25H28Cl4N4O2/c26-20-7-4-18(16-22(20)28)5-9-24(34)30-10-2-1-3-11-32-12-14-33(15-13-32)25(35)31-19-6-8-21(27)23(29)17-19/h4-9,16-17H,1-3,10-15H2,(H,30,34)(H,31,35)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194383

(CHEMBL375832 | N-(3-chloro-4-fluorophenyl)-4-(5-(3...)Show SMILES Fc1ccc(NC(=O)N2CCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C25H28Cl3FN4O2/c26-20-7-4-18(16-21(20)27)5-9-24(34)30-10-2-1-3-11-32-12-14-33(15-13-32)25(35)31-19-6-8-23(29)22(28)17-19/h4-9,16-17H,1-3,10-15H2,(H,30,34)(H,31,35)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194381
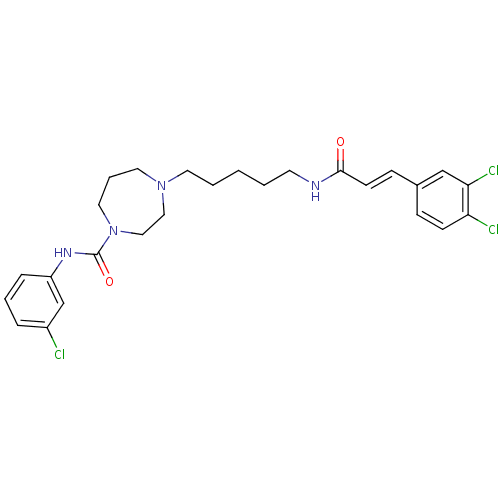
(CHEMBL373506 | N-(3-chlorophenyl)-4-(5-(3-(3,4-dic...)Show SMILES Clc1cccc(NC(=O)N2CCCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)c1 Show InChI InChI=1S/C26H31Cl3N4O2/c27-21-6-4-7-22(19-21)31-26(35)33-15-5-14-32(16-17-33)13-3-1-2-12-30-25(34)11-9-20-8-10-23(28)24(29)18-20/h4,6-11,18-19H,1-3,5,12-17H2,(H,30,34)(H,31,35)/b11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194372

(CHEMBL222729 | N-(4-chloro-3-(trifluoromethyl)phen...)Show SMILES FC(F)(F)c1cc(NC(=O)N2CCCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)ccc1Cl Show InChI InChI=1S/C27H30Cl3F3N4O2/c28-22-9-7-20(18-21(22)27(31,32)33)35-26(39)37-14-4-13-36(15-16-37)12-3-1-2-11-34-25(38)10-6-19-5-8-23(29)24(30)17-19/h5-10,17-18H,1-4,11-16H2,(H,34,38)(H,35,39)/b10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194367
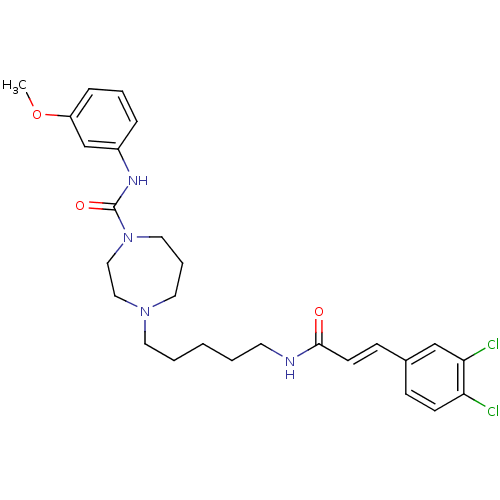
(4-(5-(3-(3,4-dichlorophenyl)acrylamido)pentyl)-N-(...)Show SMILES COc1cccc(NC(=O)N2CCCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)c1 Show InChI InChI=1S/C27H34Cl2N4O3/c1-36-23-8-5-7-22(20-23)31-27(35)33-16-6-15-32(17-18-33)14-4-2-3-13-30-26(34)12-10-21-9-11-24(28)25(29)19-21/h5,7-12,19-20H,2-4,6,13-18H2,1H3,(H,30,34)(H,31,35)/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194363

(CHEMBL221442 | N-(3,4-dichlorophenyl)-4-(5-(3-(3,4...)Show SMILES Clc1ccc(NC(=O)N2CCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C25H28Cl4N4O2/c26-20-7-4-18(16-22(20)28)5-9-24(34)30-10-2-1-3-11-32-12-14-33(15-13-32)25(35)31-19-6-8-21(27)23(29)17-19/h4-9,16-17H,1-3,10-15H2,(H,30,34)(H,31,35)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in absence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194369
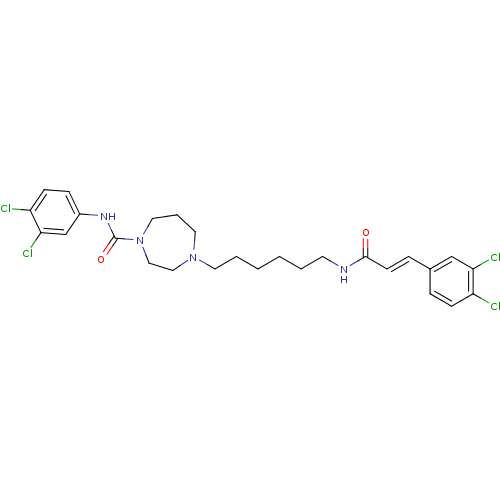
(CHEMBL376433 | N-(3,4-dichlorophenyl)-4-(6-(3-(3,4...)Show SMILES Clc1ccc(NC(=O)N2CCCN(CCCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C27H32Cl4N4O2/c28-22-9-6-20(18-24(22)30)7-11-26(36)32-12-3-1-2-4-13-34-14-5-15-35(17-16-34)27(37)33-21-8-10-23(29)25(31)19-21/h6-11,18-19H,1-5,12-17H2,(H,32,36)(H,33,37)/b11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194377
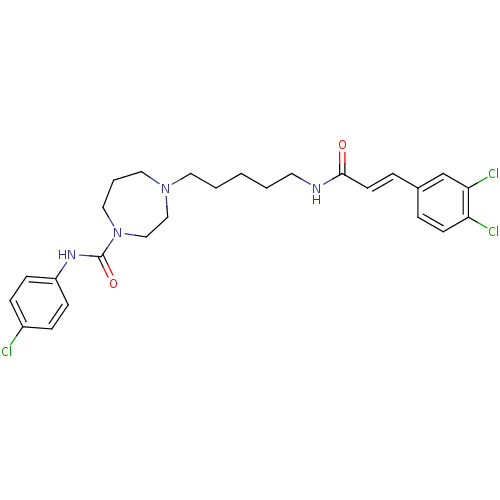
(CHEMBL220177 | N-(4-chlorophenyl)-4-(5-(3-(3,4-dic...)Show SMILES Clc1ccc(NC(=O)N2CCCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1 Show InChI InChI=1S/C26H31Cl3N4O2/c27-21-7-9-22(10-8-21)31-26(35)33-16-4-15-32(17-18-33)14-3-1-2-13-30-25(34)12-6-20-5-11-23(28)24(29)19-20/h5-12,19H,1-4,13-18H2,(H,30,34)(H,31,35)/b12-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194368
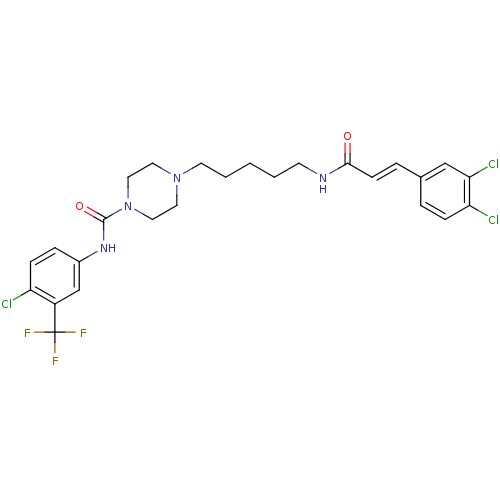
(CHEMBL222125 | N-(4-chloro-3-(trifluoromethyl)phen...)Show SMILES FC(F)(F)c1cc(NC(=O)N2CCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)ccc1Cl Show InChI InChI=1S/C26H28Cl3F3N4O2/c27-21-8-6-19(17-20(21)26(30,31)32)34-25(38)36-14-12-35(13-15-36)11-3-1-2-10-33-24(37)9-5-18-4-7-22(28)23(29)16-18/h4-9,16-17H,1-3,10-15H2,(H,33,37)(H,34,38)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194366
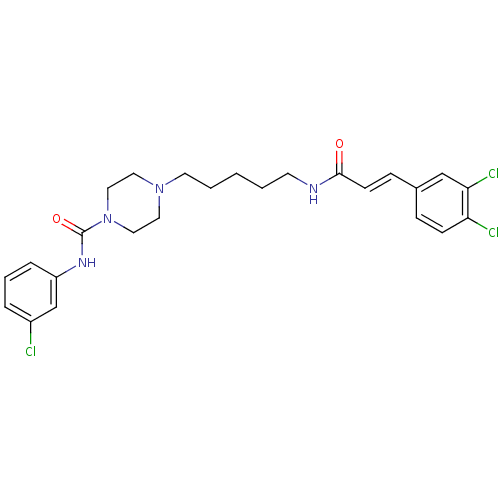
(CHEMBL373842 | N-(3-chlorophenyl)-4-(5-(3-(3,4-dic...)Show SMILES Clc1cccc(NC(=O)N2CCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)c1 Show InChI InChI=1S/C25H29Cl3N4O2/c26-20-5-4-6-21(18-20)30-25(34)32-15-13-31(14-16-32)12-3-1-2-11-29-24(33)10-8-19-7-9-22(27)23(28)17-19/h4-10,17-18H,1-3,11-16H2,(H,29,33)(H,30,34)/b10-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194385

(-N-(benzo[d][1,3]dioxol-5-yl)-4-(5-(3-(3,4-dichlor...)Show SMILES Clc1ccc(\C=C\C(=O)NCCCCCN2CCN(CC2)C(=O)Nc2ccc3OCOc3c2)cc1Cl Show InChI InChI=1S/C26H30Cl2N4O4/c27-21-7-4-19(16-22(21)28)5-9-25(33)29-10-2-1-3-11-31-12-14-32(15-13-31)26(34)30-20-6-8-23-24(17-20)36-18-35-23/h4-9,16-17H,1-3,10-15,18H2,(H,29,33)(H,30,34)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194382
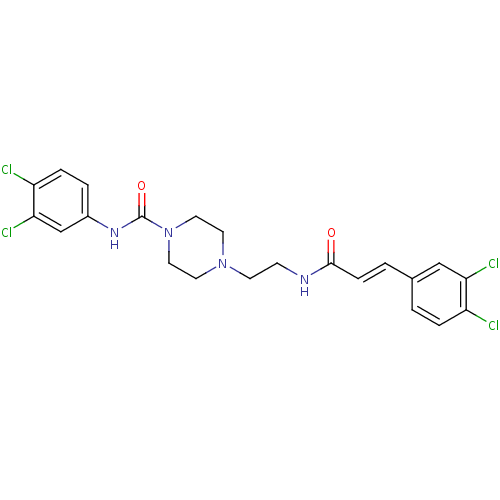
(CHEMBL223676 | N-(3,4-dichlorophenyl)-4-(2-(3-(3,4...)Show SMILES Clc1ccc(NC(=O)N2CCN(CCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C22H22Cl4N4O2/c23-17-4-1-15(13-19(17)25)2-6-21(31)27-7-8-29-9-11-30(12-10-29)22(32)28-16-3-5-18(24)20(26)14-16/h1-6,13-14H,7-12H2,(H,27,31)(H,28,32)/b6-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194371
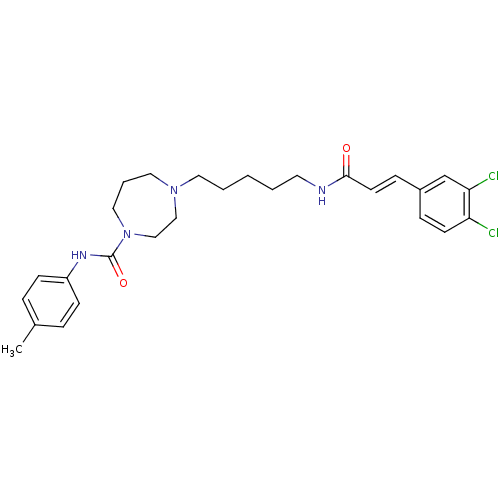
(4-(5-(3-(3,4-dichlorophenyl)acrylamido)pentyl)-N-p...)Show SMILES Cc1ccc(NC(=O)N2CCCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1 Show InChI InChI=1S/C27H34Cl2N4O2/c1-21-6-10-23(11-7-21)31-27(35)33-17-5-16-32(18-19-33)15-4-2-3-14-30-26(34)13-9-22-8-12-24(28)25(29)20-22/h6-13,20H,2-5,14-19H2,1H3,(H,30,34)(H,31,35)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194379

(4-(5-(3-(3,4-dichlorophenyl)acrylamido)pentyl)-N-(...)Show SMILES COc1cccc(NC(=O)N2CCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)c1 Show InChI InChI=1S/C26H32Cl2N4O3/c1-35-22-7-5-6-21(19-22)30-26(34)32-16-14-31(15-17-32)13-4-2-3-12-29-25(33)11-9-20-8-10-23(27)24(28)18-20/h5-11,18-19H,2-4,12-17H2,1H3,(H,29,33)(H,30,34)/b11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194378
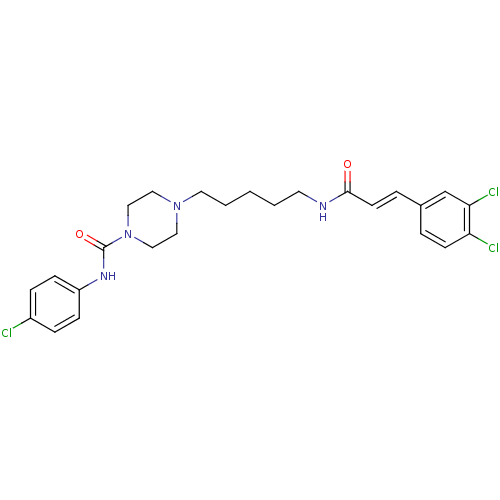
(CHEMBL222126 | N-(4-chlorophenyl)-4-(5-(3-(3,4-dic...)Show SMILES Clc1ccc(NC(=O)N2CCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1 Show InChI InChI=1S/C25H29Cl3N4O2/c26-20-6-8-21(9-7-20)30-25(34)32-16-14-31(15-17-32)13-3-1-2-12-29-24(33)11-5-19-4-10-22(27)23(28)18-19/h4-11,18H,1-3,12-17H2,(H,29,33)(H,30,34)/b11-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194373
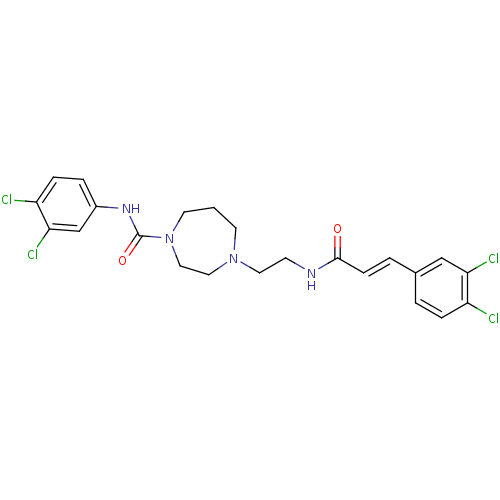
(CHEMBL222953 | N-(3,4-dichlorophenyl)-4-(2-(3-(3,4...)Show SMILES Clc1ccc(NC(=O)N2CCCN(CCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1Cl Show InChI InChI=1S/C23H24Cl4N4O2/c24-18-5-2-16(14-20(18)26)3-7-22(32)28-8-11-30-9-1-10-31(13-12-30)23(33)29-17-4-6-19(25)21(27)15-17/h2-7,14-15H,1,8-13H2,(H,28,32)(H,29,33)/b7-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194384

(4-(5-(3-(3,4-dichlorophenyl)acrylamido)pentyl)-N-p...)Show SMILES Cc1ccc(NC(=O)N2CCN(CCCCCNC(=O)\C=C\c3ccc(Cl)c(Cl)c3)CC2)cc1 Show InChI InChI=1S/C26H32Cl2N4O2/c1-20-5-9-22(10-6-20)30-26(34)32-17-15-31(16-18-32)14-4-2-3-13-29-25(33)12-8-21-7-11-23(27)24(28)19-21/h5-12,19H,2-4,13-18H2,1H3,(H,29,33)(H,30,34)/b12-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human liver GPa in presence of glucose |
Bioorg Med Chem Lett 16: 5892-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.055
BindingDB Entry DOI: 10.7270/Q2VD6Z28 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data