

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22564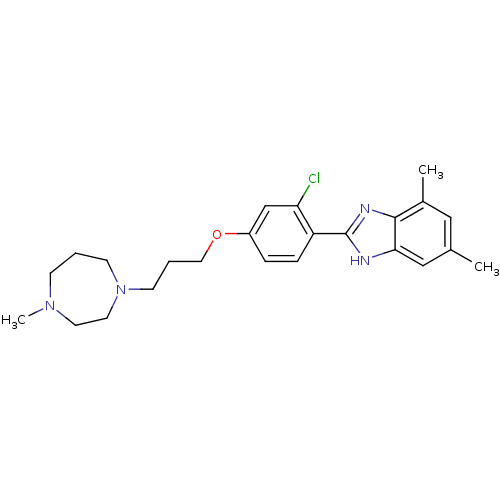 (2-arylbenzimidazole derivative, 10 | 2-{2-chloro-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22563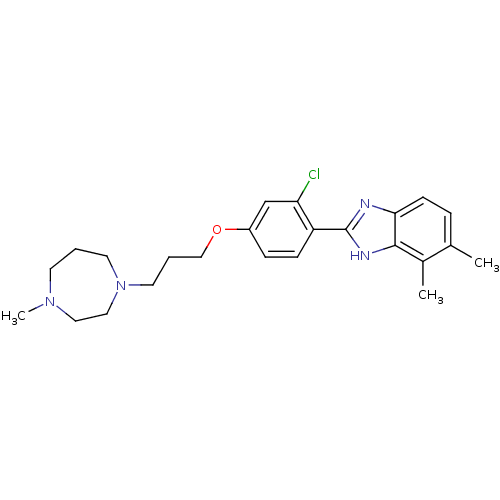 (2-arylbenzimidazole derivative, 9 | 2-{2-chloro-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22556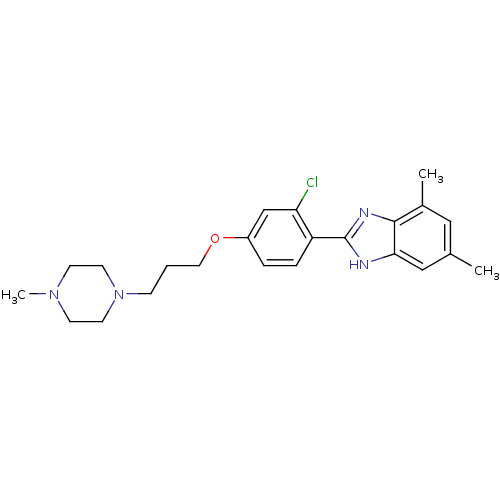 (2-arylbenzimidazole derivative, 2 | 2-{2-chloro-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | -43.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22562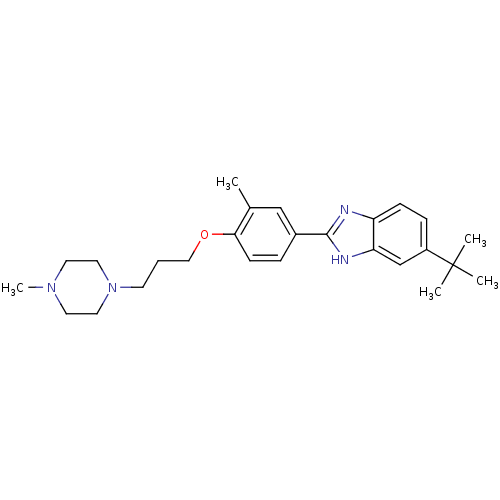 (2-arylbenzimidazole derivative, 8 | 5-tert-butyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22560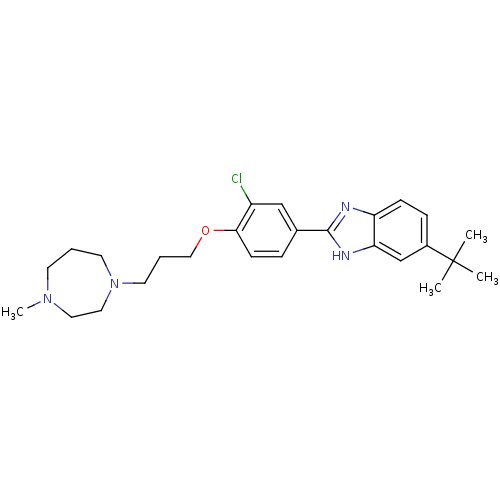 (2-arylbenzimidazole derivative, 6 | 5-tert-butyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22558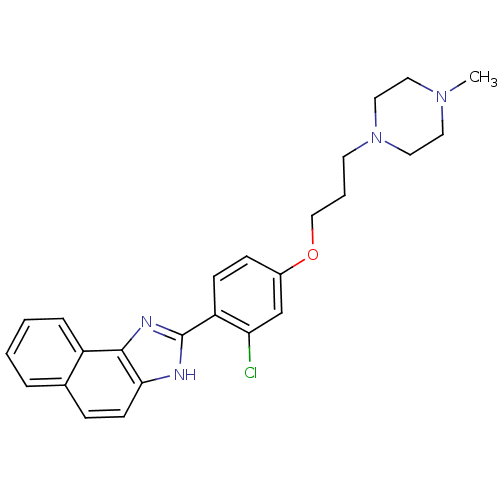 (2-arylbenzimidazole derivative, 4 | 4-{2-chloro-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22555 (2-arylbenzimidazole derivative, 1 | 2-{2-chloro-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 46 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22561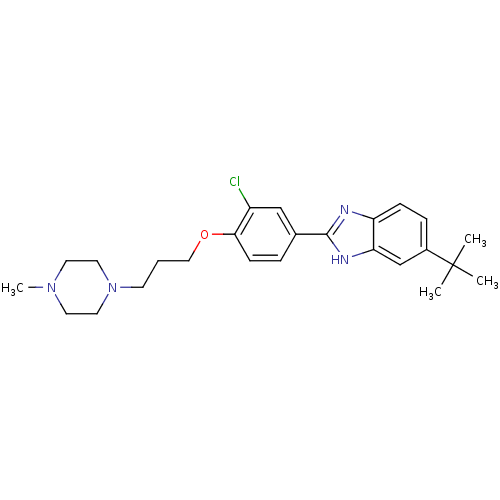 (2-arylbenzimidazole derivative, 7 | 5-tert-butyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 65 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22557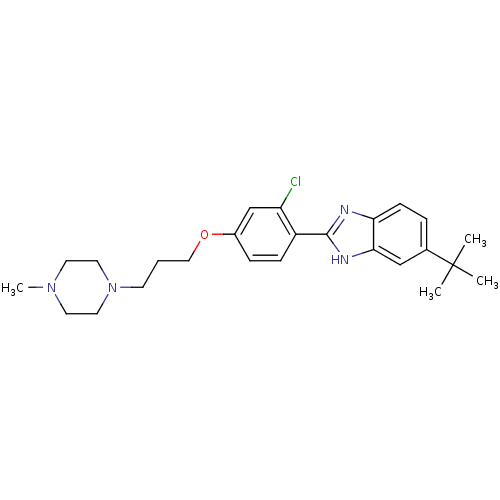 (2-arylbenzimidazole derivative, 3 | 5-tert-butyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 93 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22565 (2-arylbenzimidazole lead compound 1 | 5-chloro-2-{...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 124 | -39.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22559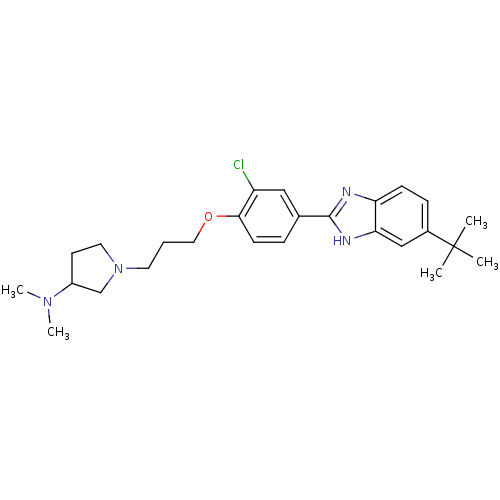 (1-{3-[4-(5-tert-butyl-1H-1,3-benzodiazol-2-yl)-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 250 | -37.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The Ki values were calculated based on an experimentally determined appropriate Kd value according to Cheng and Prusoff. | Bioorg Med Chem Lett 16: 6043-8 (2006) Article DOI: 10.1016/j.bmcl.2006.08.117 BindingDB Entry DOI: 10.7270/Q20P0XB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||