

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196604 (CHEMBL217083 | N-hydroxy-7-(naphthalen-2-yloxy)hep...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196608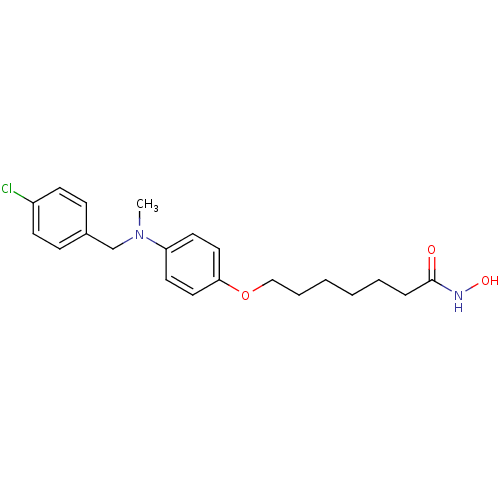 (7-(4-((4-chlorobenzyl)(methyl)amino)phenoxy)-N-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196600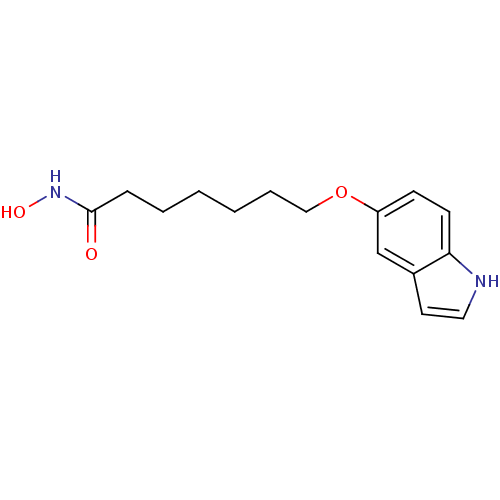 (7-(1H-indol-5-yloxy)-N-hydroxyheptanamide | CHEMBL...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196598 (7-(4-(3-(1H-indol-3-yl)prop-1-en-2-ylamino)phenyl1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196603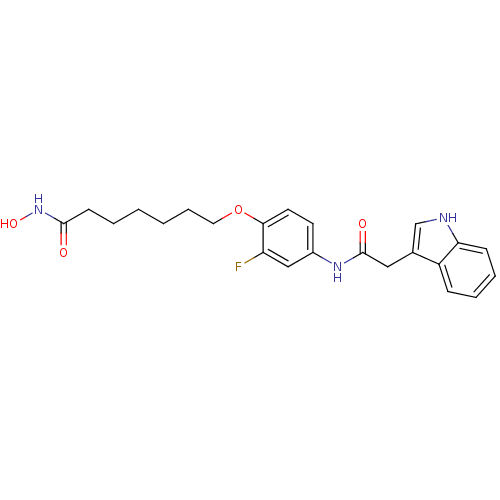 (7-(4-(2-(1H-indol-3-yl)acetamido)-2-fluorophenyl1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196605 (7-(4-(2-(1H-indol-3-yl)acetamido)phenyl1H-indol-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196594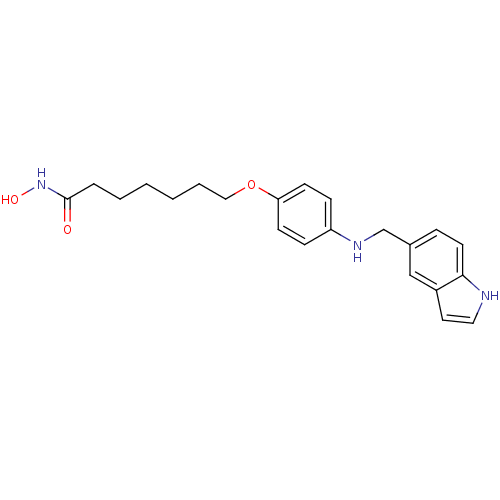 (7-(4-((1H-indol-5-yl)methylamino)phenyl1H-indol-5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196597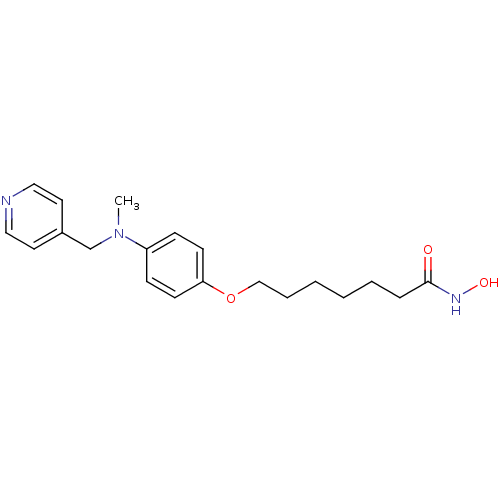 (CHEMBL384714 | N-hydroxy-7-(4-(methyl(pyridin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196607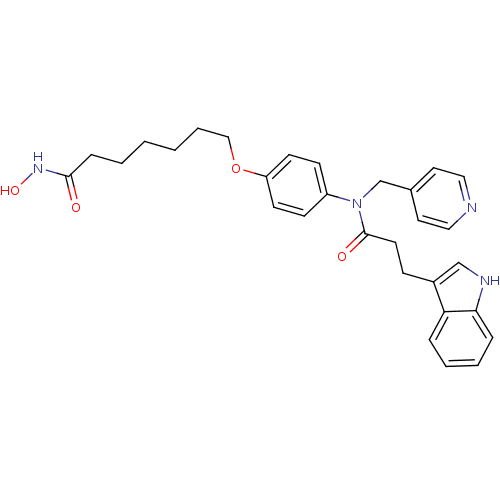 (7-(4-(3-(1H-indol-3-yl)-N-(pyridin-4-ylmethyl)prop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196609 (7-(4-(3-(1H-indol-3-yl)propanamido)phenyl1H-indol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196602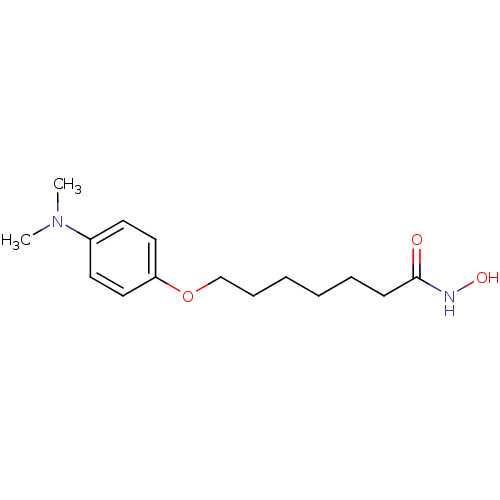 (7-(4-(dimethylamino)phenoxy)-N-hydroxyheptanamide ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196593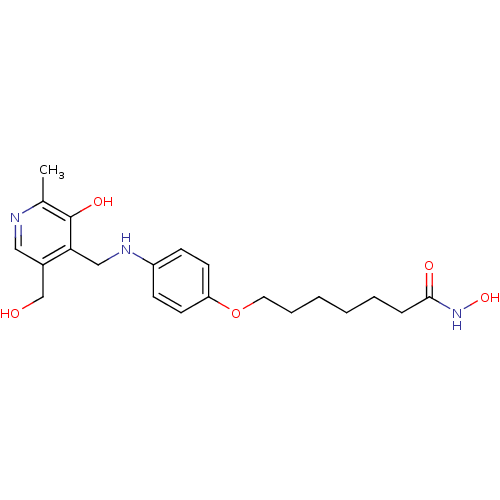 (CHEMBL387042 | N-hydroxy-7-(4-((3-hydroxy-5-(hydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196596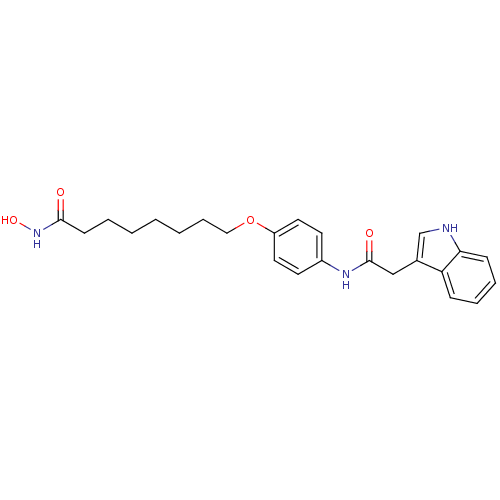 (8-(4-(2-(1H-indol-3-yl)acetamido)phenyl1H-indol-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196601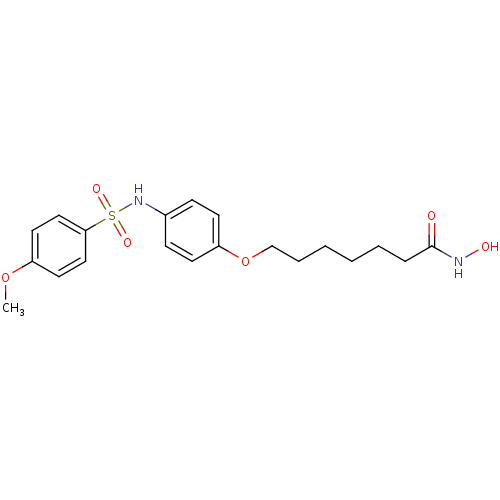 (CHEMBL217778 | N-hydroxy-7-(4-(4-methoxyphenylsulf...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196606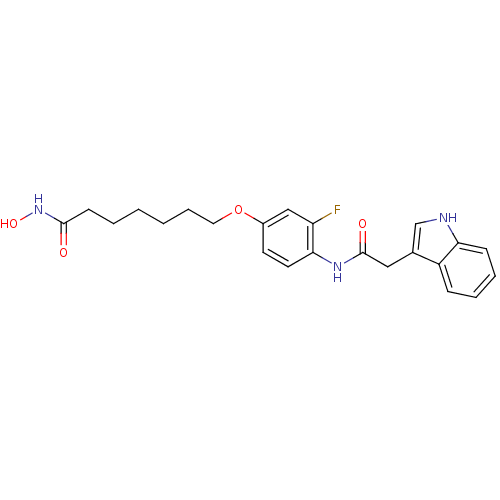 (7-(4-(2-(1H-indol-3-yl)acetamido)-3-fluorophenyl1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196595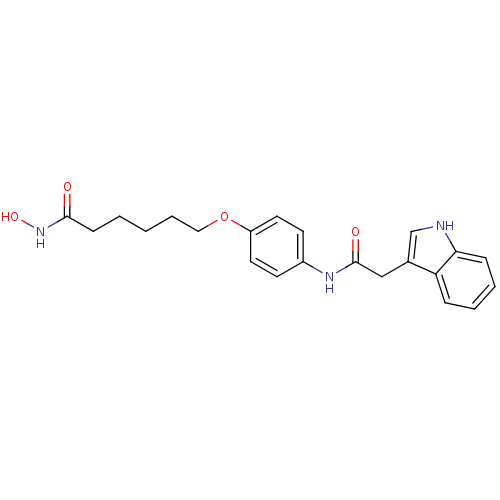 (6-(4-(2-(1H-indol-3-yl)acetamido)phenyl1H-indol-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50196599 (7-(3-fluoro-4-((3-hydroxy-5-(hydroxymethyl)-2-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description In vitro inhibition of histone deacetylase activity using HeLa cell nuclear extract as enzyme source | Bioorg Med Chem Lett 17: 136-41 (2006) Article DOI: 10.1016/j.bmcl.2006.09.085 BindingDB Entry DOI: 10.7270/Q2B56JDQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||