 Found 19 hits of Enzyme Inhibition Constant Data
Found 19 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25052
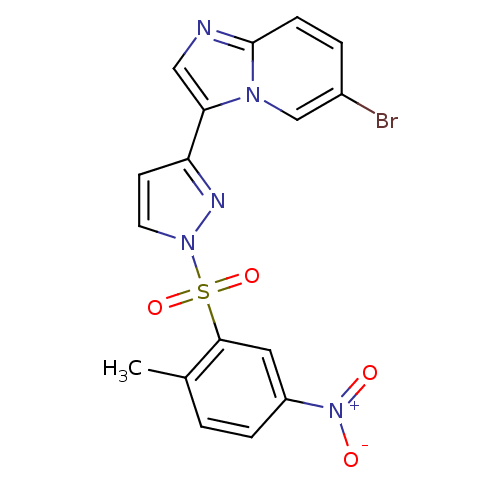
(3-{6-bromoimidazo[1,2-a]pyridin-3-yl}-1-[(2-methyl...)Show SMILES Cc1ccc(cc1S(=O)(=O)n1ccc(n1)-c1cnc2ccc(Br)cn12)[N+]([O-])=O Show InChI InChI=1S/C17H12BrN5O4S/c1-11-2-4-13(23(24)25)8-16(11)28(26,27)22-7-6-14(20-22)15-9-19-17-5-3-12(18)10-21(15)17/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25050

(3-{6-chloro-2-methylimidazo[1,2-a]pyridin-3-yl}-1-...)Show SMILES Cc1nc2ccc(Cl)cn2c1-c1ccn(n1)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C18H14ClN5O4S/c1-11-3-5-14(24(25)26)9-16(11)29(27,28)23-8-7-15(21-23)18-12(2)20-17-6-4-13(19)10-22(17)18/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25056
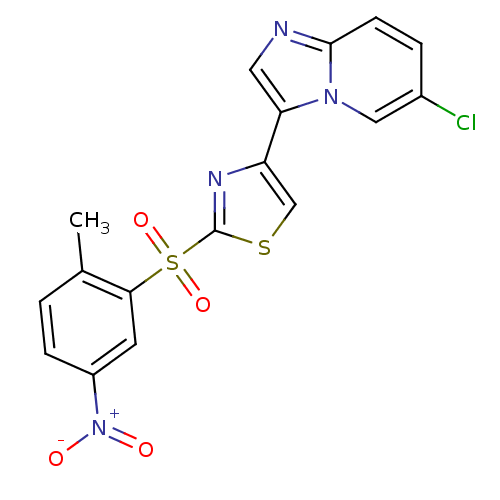
(4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methy...)Show SMILES Cc1ccc(cc1S(=O)(=O)c1nc(cs1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN4O4S2/c1-10-2-4-12(22(23)24)6-15(10)28(25,26)17-20-13(9-27-17)14-7-19-16-5-3-11(18)8-21(14)16/h2-9H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25030

(3-{6-bromo-2-methylimidazo[1,2-a]pyridin-3-yl}-1-[...)Show SMILES Cc1nc2ccc(Br)cn2c1-c1ccn(n1)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C18H14BrN5O4S/c1-11-3-5-14(24(25)26)9-16(11)29(27,28)23-8-7-15(21-23)18-12(2)20-17-6-4-13(19)10-22(17)18/h3-10H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25057
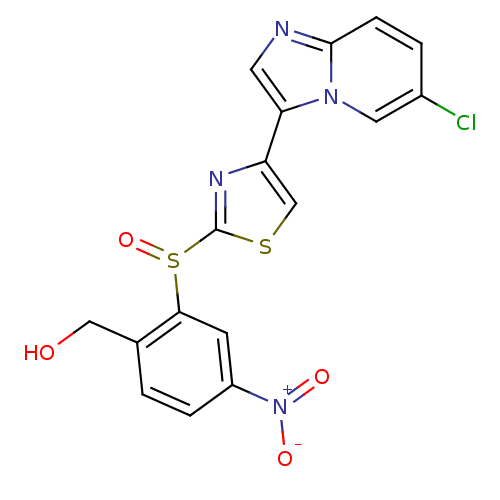
(imidazo[1,2-a]pyridine derivative, 14b | {2-[(4-{6...)Show SMILES OCc1ccc(cc1S(=O)c1nc(cs1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN4O4S2/c18-11-2-4-16-19-6-14(21(16)7-11)13-9-27-17(20-13)28(26)15-5-12(22(24)25)3-1-10(15)8-23/h1-7,9,23H,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25055

(4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methy...)Show SMILES Cc1ccc(cc1S(=O)c1nc(cs1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN4O3S2/c1-10-2-4-12(22(23)24)6-15(10)27(25)17-20-13(9-26-17)14-7-19-16-5-3-11(18)8-21(14)16/h2-9H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25054

(4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methy...)Show SMILES Cc1ccc(cc1Sc1nc(cs1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN4O2S2/c1-10-2-4-12(22(23)24)6-15(10)26-17-20-13(9-25-17)14-7-19-16-5-3-11(18)8-21(14)16/h2-9H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM25056

(4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methy...)Show SMILES Cc1ccc(cc1S(=O)(=O)c1nc(cs1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN4O4S2/c1-10-2-4-12(22(23)24)6-15(10)28(25,26)17-20-13(9-27-17)14-7-19-16-5-3-11(18)8-21(14)16/h2-9H,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25053
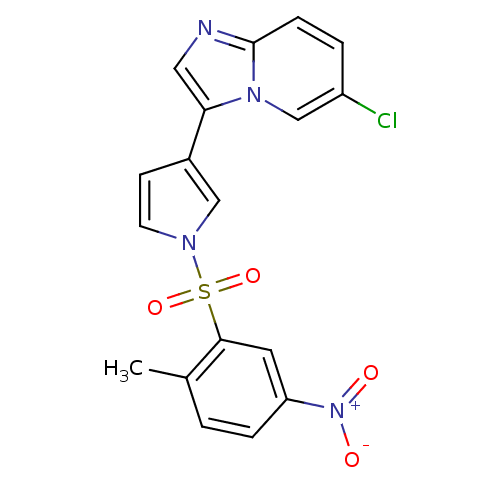
(3-{6-chloroimidazo[1,2-a]pyridin-3-yl}-1-[(2-methy...)Show SMILES Cc1ccc(cc1S(=O)(=O)n1ccc(c1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O Show InChI InChI=1S/C18H13ClN4O4S/c1-12-2-4-15(23(24)25)8-17(12)28(26,27)21-7-6-13(10-21)16-9-20-18-5-3-14(19)11-22(16)18/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM25056

(4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methy...)Show SMILES Cc1ccc(cc1S(=O)(=O)c1nc(cs1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN4O4S2/c1-10-2-4-12(22(23)24)6-15(10)28(25,26)17-20-13(9-27-17)14-7-19-16-5-3-11(18)8-21(14)16/h2-9H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25056

(4-{6-chloroimidazo[1,2-a]pyridin-3-yl}-2-[(2-methy...)Show SMILES Cc1ccc(cc1S(=O)(=O)c1nc(cs1)-c1cnc2ccc(Cl)cn12)[N+]([O-])=O Show InChI InChI=1S/C17H11ClN4O4S2/c1-10-2-4-12(22(23)24)6-15(10)28(25,26)17-20-13(9-27-17)14-7-19-16-5-3-11(18)8-21(14)16/h2-9H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25049

(3-{6-chloro-2-methylimidazo[1,2-a]pyridin-3-yl}-1-...)Show SMILES Cc1nc2ccc(Cl)cn2c1-c1ccn(n1)S(=O)(=O)c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C17H12ClN5O4S/c1-11-17(21-10-12(18)5-6-16(21)19-11)15-7-8-22(20-15)28(26,27)14-4-2-3-13(9-14)23(24)25/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25029
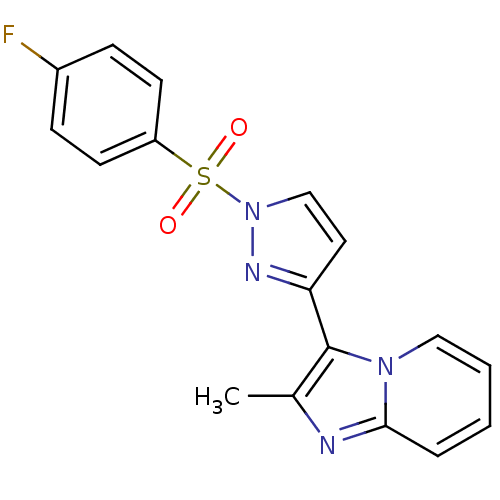
(1-[(4-fluorobenzene)sulfonyl]-3-{2-methylimidazo[1...)Show SMILES Cc1nc2ccccn2c1-c1ccn(n1)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C17H13FN4O2S/c1-12-17(21-10-3-2-4-16(21)19-12)15-9-11-22(20-15)25(23,24)14-7-5-13(18)6-8-14/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
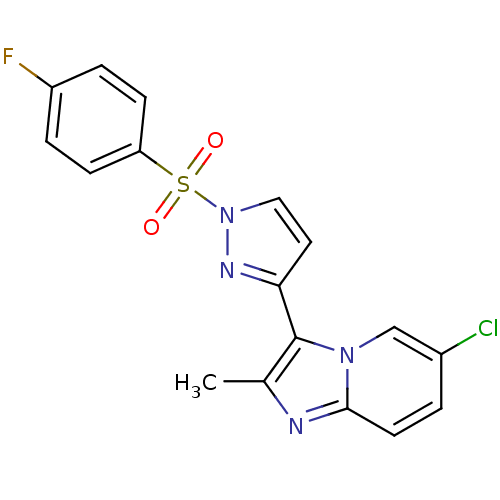
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25047
(3-{6-chloro-2-methylimidazo[1,2-a]pyridin-3-yl}-1-...)Show SMILES Cc1nc2ccc(Cl)cn2c1-c1ccn(n1)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C17H12ClFN4O2S/c1-11-17(22-10-12(18)2-7-16(22)20-11)15-8-9-23(21-15)26(24,25)14-5-3-13(19)4-6-14/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit beta
(Homo sapiens (Human)) | BDBM12915

(2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one |...)Show InChI InChI=1S/C19H17NO3/c21-17-13-18(20-9-11-22-12-10-20)23-19-15(7-4-8-16(17)19)14-5-2-1-3-6-14/h1-8,13H,9-12H2 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25048
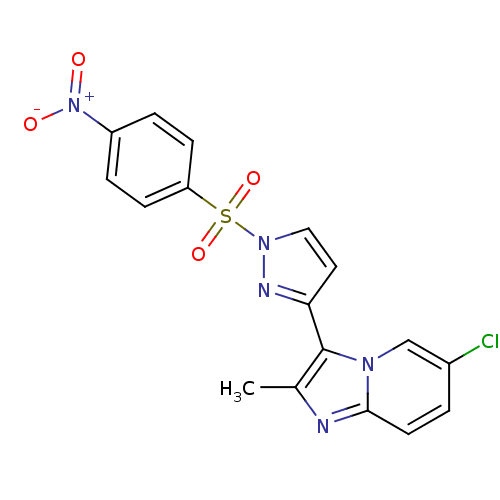
(3-{6-chloro-2-methylimidazo[1,2-a]pyridin-3-yl}-1-...)Show SMILES Cc1nc2ccc(Cl)cn2c1-c1ccn(n1)S(=O)(=O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C17H12ClN5O4S/c1-11-17(21-10-12(18)2-7-16(21)19-11)15-8-9-22(20-15)28(26,27)14-5-3-13(4-6-14)23(24)25/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Astellas Pharma Inc.
| Assay Description
The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... |
Bioorg Med Chem 15: 403-12 (2007)
Article DOI: 10.1016/j.bmc.2006.09.047
BindingDB Entry DOI: 10.7270/Q2SJ1HXZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data