 Found 10 hits of Enzyme Inhibition Constant Data
Found 10 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18885
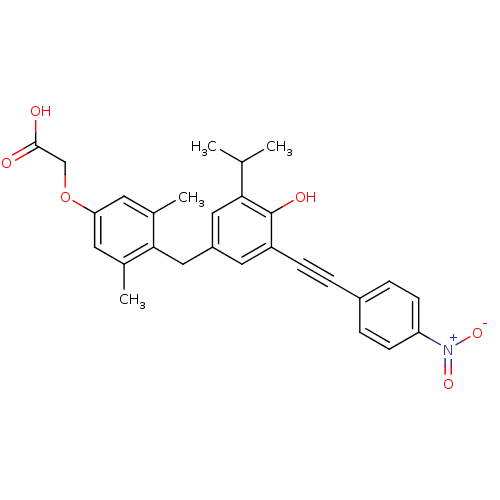
(2-[4-({4-hydroxy-3-[2-(4-nitrophenyl)ethynyl]-5-(p...)Show SMILES CC(C)c1cc(Cc2c(C)cc(OCC(O)=O)cc2C)cc(C#Cc2ccc(cc2)[N+]([O-])=O)c1O Show InChI InChI=1S/C28H27NO6/c1-17(2)25-14-21(15-26-18(3)11-24(12-19(26)4)35-16-27(30)31)13-22(28(25)32)8-5-20-6-9-23(10-7-20)29(33)34/h6-7,9-14,17,32H,15-16H2,1-4H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
J Med Chem 49: 6635-7 (2006)
Article DOI: 10.1021/jm060521i
BindingDB Entry DOI: 10.7270/Q23B5XDF |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18886
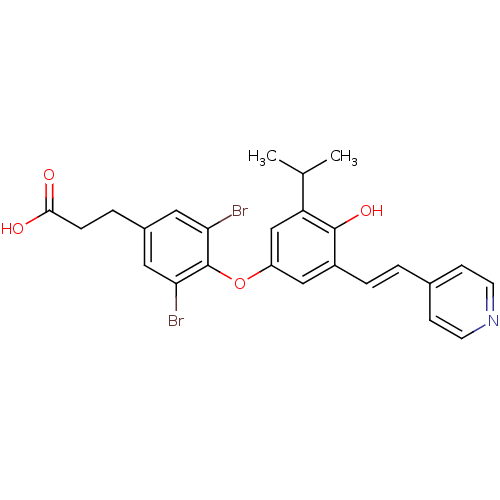
(3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)-5-[(E)...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CCC(O)=O)cc2Br)cc(\C=C\c2ccncc2)c1O Show InChI InChI=1S/C25H23Br2NO4/c1-15(2)20-14-19(13-18(24(20)31)5-3-16-7-9-28-10-8-16)32-25-21(26)11-17(12-22(25)27)4-6-23(29)30/h3,5,7-15,31H,4,6H2,1-2H3,(H,29,30)/b5-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 22 | n/a | 32 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to i... |
J Med Chem 49: 6635-7 (2006)
Article DOI: 10.1021/jm060521i
BindingDB Entry DOI: 10.7270/Q23B5XDF |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18884
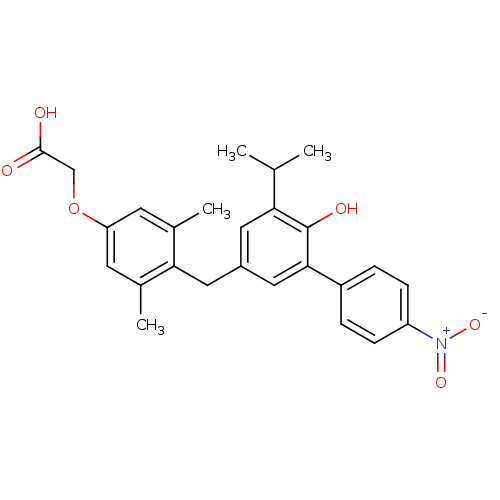
(2-(4-{[4-hydroxy-3-(4-nitrophenyl)-5-(propan-2-yl)...)Show SMILES CC(C)c1cc(Cc2c(C)cc(OCC(O)=O)cc2C)cc(c1O)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C26H27NO6/c1-15(2)22-11-18(12-23-16(3)9-21(10-17(23)4)33-14-25(28)29)13-24(26(22)30)19-5-7-20(8-6-19)27(31)32/h5-11,13,15,30H,12,14H2,1-4H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
J Med Chem 49: 6635-7 (2006)
Article DOI: 10.1021/jm060521i
BindingDB Entry DOI: 10.7270/Q23B5XDF |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18886

(3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)-5-[(E)...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CCC(O)=O)cc2Br)cc(\C=C\c2ccncc2)c1O Show InChI InChI=1S/C25H23Br2NO4/c1-15(2)20-14-19(13-18(24(20)31)5-3-16-7-9-28-10-8-16)32-25-21(26)11-17(12-22(25)27)4-6-23(29)30/h3,5,7-15,31H,4,6H2,1-2H3,(H,29,30)/b5-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 36 | n/a | 32 | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... |
J Med Chem 49: 6635-7 (2006)
Article DOI: 10.1021/jm060521i
BindingDB Entry DOI: 10.7270/Q23B5XDF |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18885

(2-[4-({4-hydroxy-3-[2-(4-nitrophenyl)ethynyl]-5-(p...)Show SMILES CC(C)c1cc(Cc2c(C)cc(OCC(O)=O)cc2C)cc(C#Cc2ccc(cc2)[N+]([O-])=O)c1O Show InChI InChI=1S/C28H27NO6/c1-17(2)25-14-21(15-26-18(3)11-24(12-19(26)4)35-16-27(30)31)13-22(28(25)32)8-5-20-6-9-23(10-7-20)29(33)34/h6-7,9-14,17,32H,15-16H2,1-4H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 49: 6635-7 (2006)
Article DOI: 10.1021/jm060521i
BindingDB Entry DOI: 10.7270/Q23B5XDF |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18887
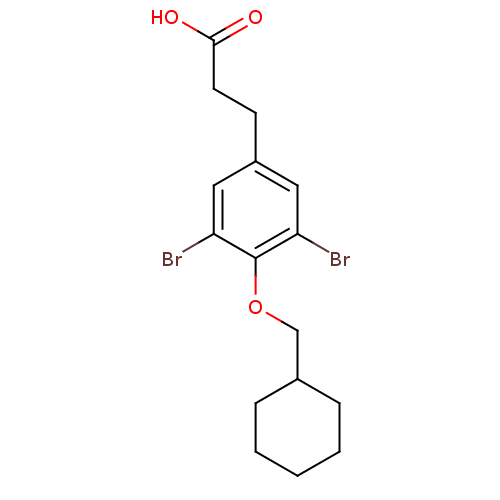
(3,5-Dibromo-4-alkoxyphenylalkanoic Acid, 9k | 3-[3...)Show InChI InChI=1S/C16H20Br2O3/c17-13-8-12(6-7-15(19)20)9-14(18)16(13)21-10-11-4-2-1-3-5-11/h8-9,11H,1-7,10H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
J Med Chem 49: 6635-7 (2006)
Article DOI: 10.1021/jm060521i
BindingDB Entry DOI: 10.7270/Q23B5XDF |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18884

(2-(4-{[4-hydroxy-3-(4-nitrophenyl)-5-(propan-2-yl)...)Show SMILES CC(C)c1cc(Cc2c(C)cc(OCC(O)=O)cc2C)cc(c1O)-c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C26H27NO6/c1-15(2)22-11-18(12-23-16(3)9-21(10-17(23)4)33-14-25(28)29)13-24(26(22)30)19-5-7-20(8-6-19)27(31)32/h5-11,13,15,30H,12,14H2,1-4H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 49: 6635-7 (2006)
Article DOI: 10.1021/jm060521i
BindingDB Entry DOI: 10.7270/Q23B5XDF |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18887

(3,5-Dibromo-4-alkoxyphenylalkanoic Acid, 9k | 3-[3...)Show InChI InChI=1S/C16H20Br2O3/c17-13-8-12(6-7-15(19)20)9-14(18)16(13)21-10-11-4-2-1-3-5-11/h8-9,11H,1-7,10H2,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 49: 6635-7 (2006)
Article DOI: 10.1021/jm060521i
BindingDB Entry DOI: 10.7270/Q23B5XDF |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18883

(3,5-dibromo-4-[4-hydroxy-3,5-bis(propan-2-yl)pheno...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(cc2Br)C(O)=O)cc(C(C)C)c1O Show InChI InChI=1S/C19H20Br2O4/c1-9(2)13-7-12(8-14(10(3)4)17(13)22)25-18-15(20)5-11(19(23)24)6-16(18)21/h5-10,22H,1-4H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. |
J Med Chem 49: 6635-7 (2006)
Article DOI: 10.1021/jm060521i
BindingDB Entry DOI: 10.7270/Q23B5XDF |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18883

(3,5-dibromo-4-[4-hydroxy-3,5-bis(propan-2-yl)pheno...)Show SMILES CC(C)c1cc(Oc2c(Br)cc(cc2Br)C(O)=O)cc(C(C)C)c1O Show InChI InChI=1S/C19H20Br2O4/c1-9(2)13-7-12(8-14(10(3)4)17(13)22)25-18-15(20)5-11(19(23)24)6-16(18)21/h5-10,22H,1-4H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB
| Assay Description
IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. |
J Med Chem 49: 6635-7 (2006)
Article DOI: 10.1021/jm060521i
BindingDB Entry DOI: 10.7270/Q23B5XDF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data