

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008071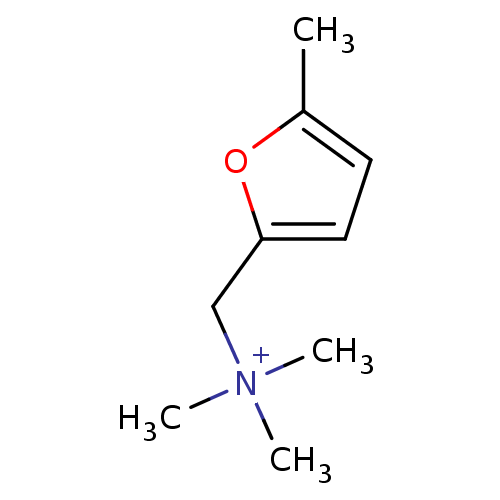 (5-methylfurmethide | CHEMBL92387 | Trimethyl-(5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Central presynaptic activity as effect on electrically evoked acetylcholine release from rat hippocampal slices (M2 model) | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008068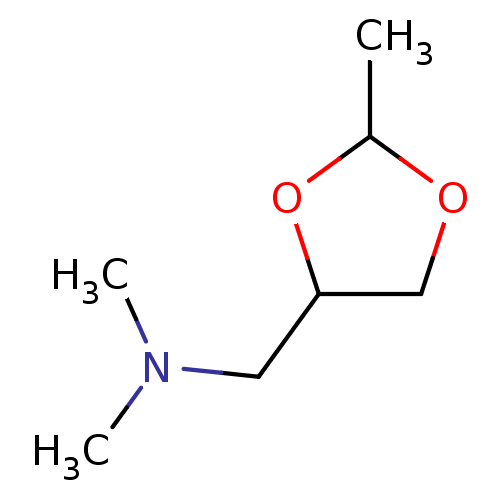 (CHEMBL327819 | Dimethyl-(2-methyl-[1,3]dioxolan-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50006243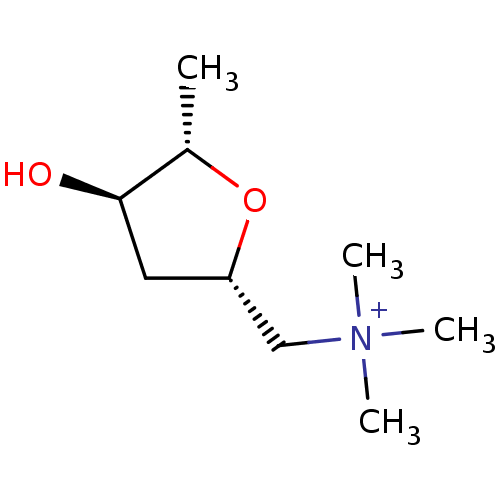 (((2S,4R,5S)-4-Hydroxy-5-methyl-tetrahydro-furan-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Central presynaptic activity as effect on electrically evoked acetylcholine release from rat hippocampal slices (M2 model) | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM46858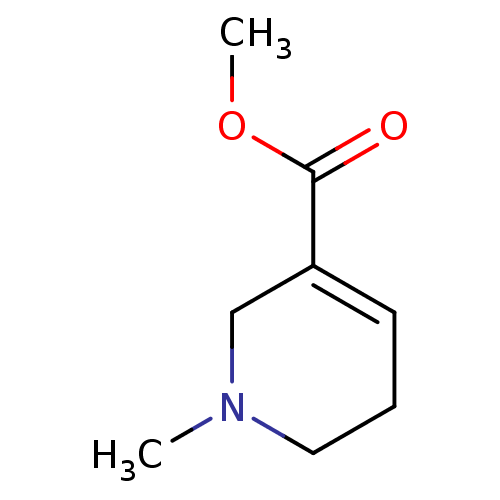 (1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Central presynaptic activity as effect on electrically evoked acetylcholine release from rat hippocampal slices (M2 model) | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008081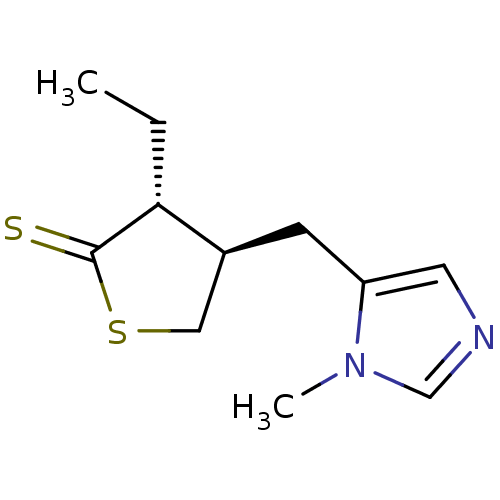 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008081 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-dihydr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008070 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Central presynaptic activity as effect on electrically evoked acetylcholine release from rat hippocampal slices (M2 model) | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008072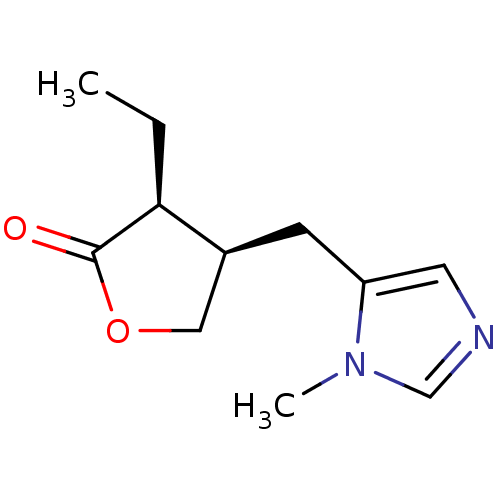 ((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Central presynaptic activity as effect on electrically evoked acetylcholine release from rat hippocampal slices (M2 model) | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008064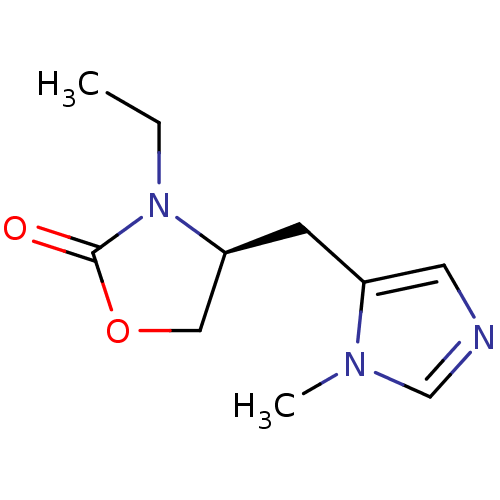 ((S)-3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008077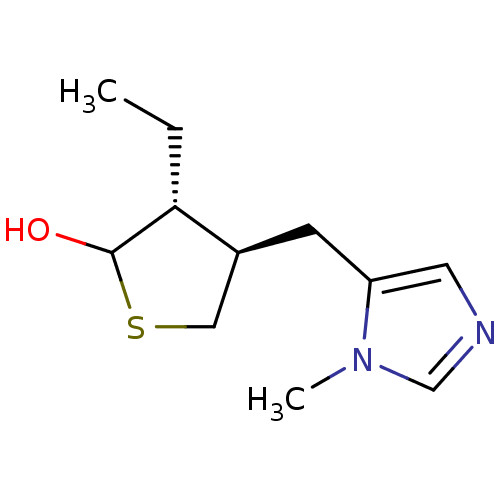 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-tetrah...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 285 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008064 ((S)-3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-ox...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008078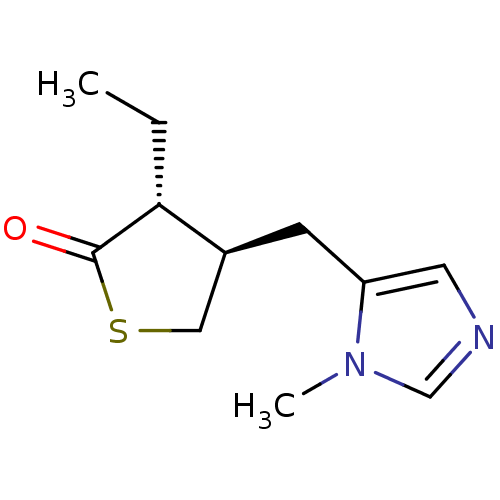 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008077 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Central presynaptic activity as effect on electrically evoked acetylcholine release from rat hippocampal slices (M2 model) | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008075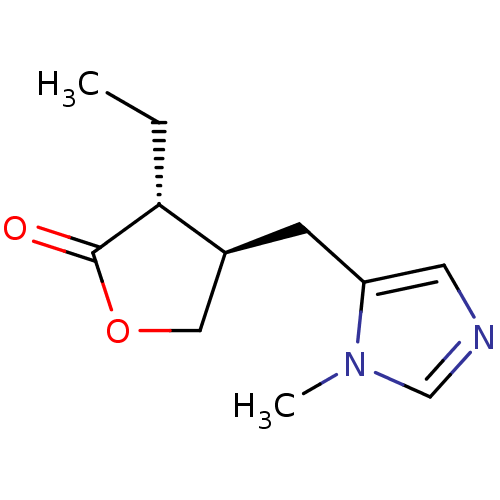 ((+)-isopilocarpine | (3R,4R)-3-ethyl-4-[(1-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008070 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-dihydr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 435 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008080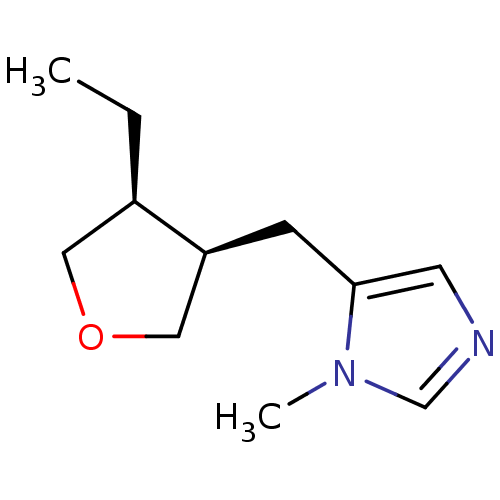 (5-(4-Ethyl-tetrahydro-furan-3-ylmethyl)-1-methyl-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 445 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Central presynaptic activity as effect on electrically evoked acetylcholine release from rat hippocampal slices (M2 model) | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008079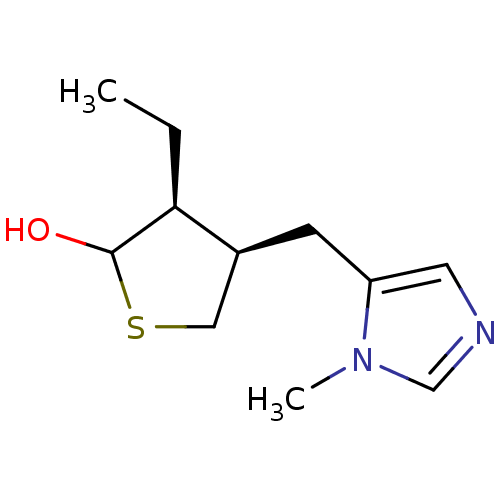 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-tetrah...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 695 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008079 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 705 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008078 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-dihydr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 775 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008067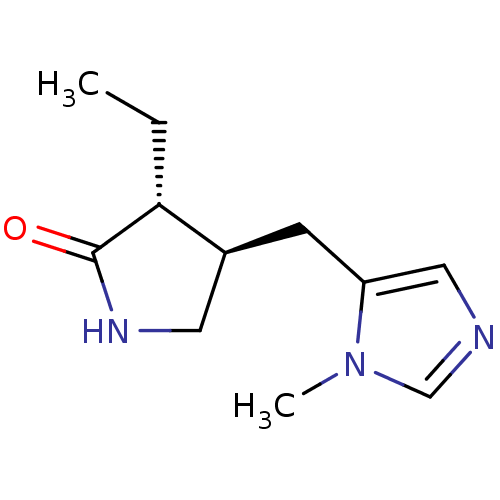 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-pyrrol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008074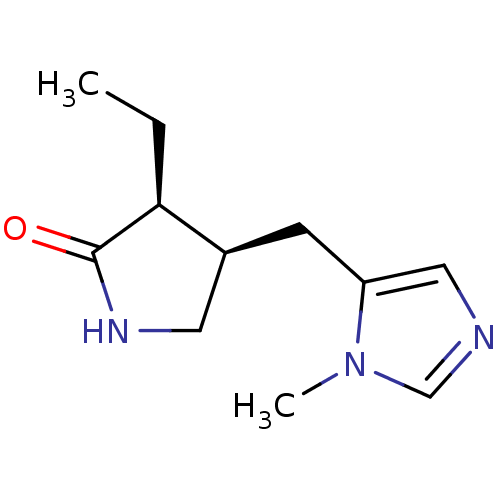 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-pyrrol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 845 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008072 ((+)-pilocarpine | (3S,4R)-3-ethyl-4-[(1-methyl-1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008071 (5-methylfurmethide | CHEMBL92387 | Trimethyl-(5-me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM46858 (1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008075 ((+)-isopilocarpine | (3R,4R)-3-ethyl-4-[(1-methyl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008080 (5-(4-Ethyl-tetrahydro-furan-3-ylmethyl)-1-methyl-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008068 (CHEMBL327819 | Dimethyl-(2-methyl-[1,3]dioxolan-4-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008073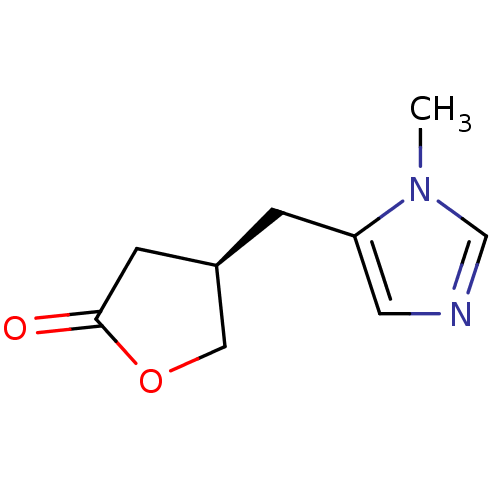 (4-(3-Methyl-3H-imidazol-4-ylmethyl)-dihydro-furan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008076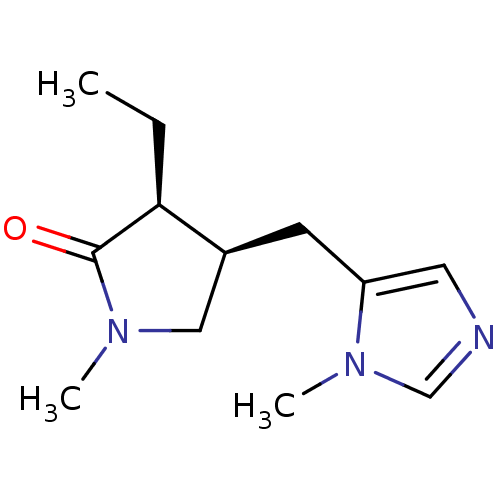 (3-Ethyl-1-methyl-4-(3-methyl-3H-imidazol-4-ylmethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008074 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-pyrrol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50006243 (((2S,4R,5S)-4-Hydroxy-5-methyl-tetrahydro-furan-2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008067 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-pyrrol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008073 (4-(3-Methyl-3H-imidazol-4-ylmethyl)-dihydro-furan-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008069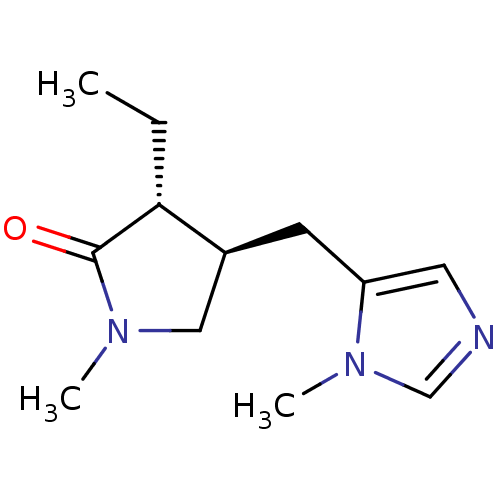 (3-Ethyl-1-methyl-4-(3-methyl-3H-imidazol-4-ylmethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008069 (3-Ethyl-1-methyl-4-(3-methyl-3H-imidazol-4-ylmethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Central presynaptic activity as effect on electrically evoked acetylcholine release from rat hippocampal slices (M2 model) | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008076 (3-Ethyl-1-methyl-4-(3-methyl-3H-imidazol-4-ylmethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Ability to displace [3H]-pirenzepine (pir) from muscarinic acetylcholine receptor M1 in rat cortical tissue. | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50008065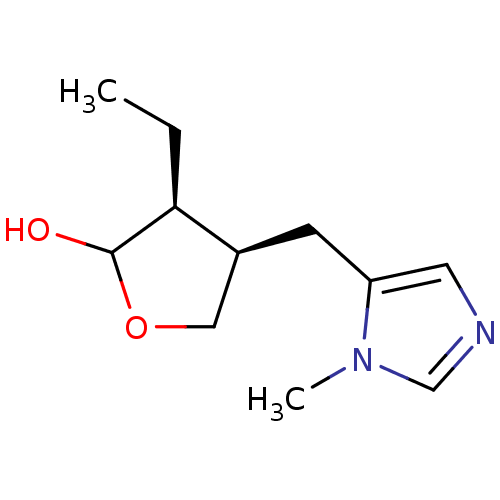 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Central presynaptic activity as effect on electrically evoked acetylcholine release from rat hippocampal slices (M2 model) | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50008065 (3-Ethyl-4-(3-methyl-3H-imidazol-4-ylmethyl)-tetrah...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd. Curated by ChEMBL | Assay Description Compound was tested for its ability to displace [3H]-pirenzepine (pir) from M1 Muscarinic receptor in rat cortical tissue | J Med Chem 35: 15-27 (1992) BindingDB Entry DOI: 10.7270/Q29022RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||