 Found 78 hits of Enzyme Inhibition Constant Data
Found 78 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206910
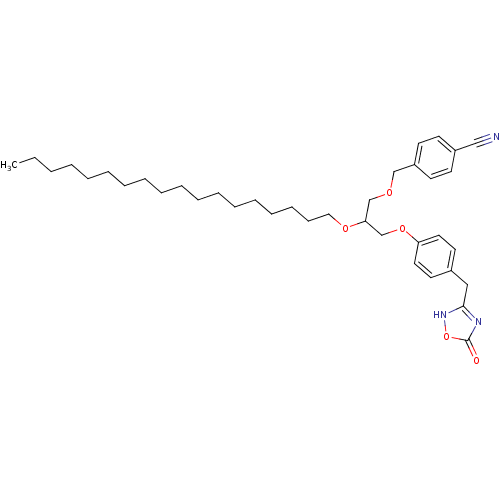
((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C38H55N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26-44-36(30-43-29-34-20-18-33(28-39)19-21-34)31-45-35-24-22-32(23-25-35)27-37-40-38(42)46-41-37/h18-25,36H,2-17,26-27,29-31H2,1H3,(H,40,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055371
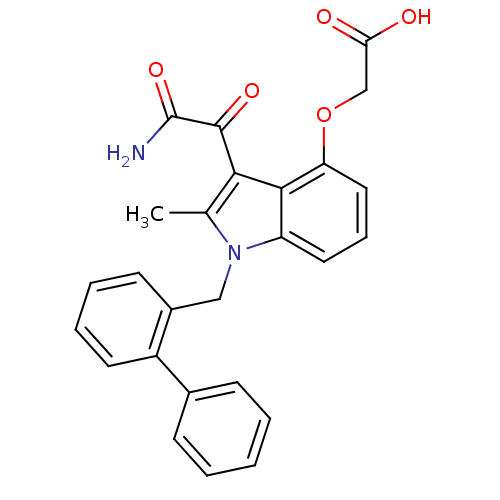
((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50055371

((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206911

((+/+)-1-O-decyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCCCCCCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H60N2O5/c1-3-5-7-9-11-13-14-15-16-18-20-22-28-40-33(29-39-27-21-19-17-12-10-8-6-4-2)30-41-32-25-23-31(24-26-32)34-36-35(38)42-37-34/h23-26,33H,3-22,27-30H2,1-2H3,(H,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206913
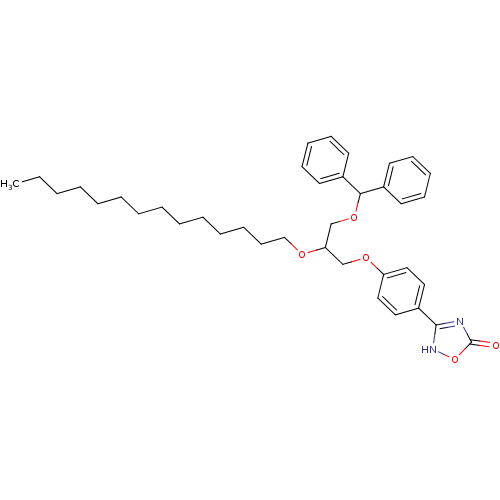
((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COC(c1ccccc1)c1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C38H50N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-19-28-42-35(29-43-34-26-24-33(25-27-34)37-39-38(41)45-40-37)30-44-36(31-20-15-13-16-21-31)32-22-17-14-18-23-32/h13-18,20-27,35-36H,2-12,19,28-30H2,1H3,(H,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206912
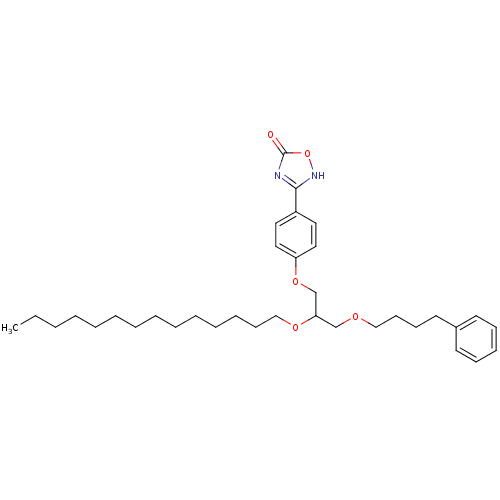
((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H52N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-17-27-40-33(28-39-26-18-16-21-30-19-14-13-15-20-30)29-41-32-24-22-31(23-25-32)34-36-35(38)42-37-34/h13-15,19-20,22-25,33H,2-12,16-18,21,26-29H2,1H3,(H,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206905

((S)-3-(4-(2-(tetradecyloxy)-3-(trityloxy)propoxy)p...)Show SMILES CCCCCCCCCCCCCCO[C@@H](COc1ccc(cc1)-c1nc(=O)o[nH]1)COC(c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C44H54N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-22-33-48-41(34-49-40-31-29-36(30-32-40)42-45-43(47)51-46-42)35-50-44(37-23-16-13-17-24-37,38-25-18-14-19-26-38)39-27-20-15-21-28-39/h13-21,23-32,41H,2-12,22,33-35H2,1H3,(H,45,46,47)/t41-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM23771
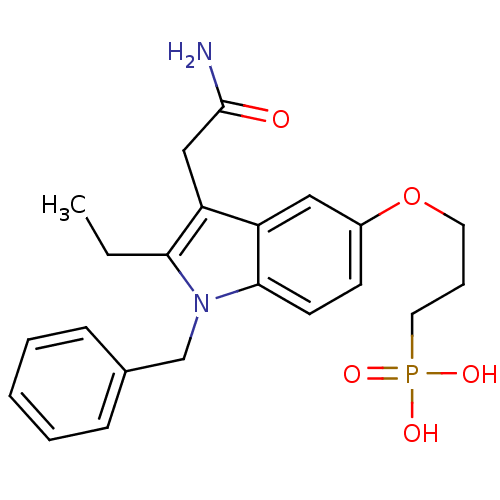
((3-{[1-benzyl-3-(carbamoylmethyl)-2-ethyl-1H-indol...)Show SMILES CCc1c(CC(N)=O)c2cc(OCCCP(O)(O)=O)ccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H27N2O5P/c1-2-20-19(14-22(23)25)18-13-17(29-11-6-12-30(26,27)28)9-10-21(18)24(20)15-16-7-4-3-5-8-16/h3-5,7-10,13H,2,6,11-12,14-15H2,1H3,(H2,23,25)(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50206911

((+/+)-1-O-decyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCCCCCCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H60N2O5/c1-3-5-7-9-11-13-14-15-16-18-20-22-28-40-33(29-39-27-21-19-17-12-10-8-6-4-2)30-41-32-25-23-31(24-26-32)34-36-35(38)42-37-34/h23-26,33H,3-22,27-30H2,1-2H3,(H,36,37,38) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50206920

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(cc1)-c1nc(=O)o[nH]1)COC(c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C44H54N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-22-33-48-41(34-49-40-31-29-36(30-32-40)42-45-43(47)51-46-42)35-50-44(37-23-16-13-17-24-37,38-25-18-14-19-26-38)39-27-20-15-21-28-39/h13-21,23-32,41H,2-12,22,33-35H2,1H3,(H,45,46,47) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206920

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(cc1)-c1nc(=O)o[nH]1)COC(c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C44H54N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-22-33-48-41(34-49-40-31-29-36(30-32-40)42-45-43(47)51-46-42)35-50-44(37-23-16-13-17-24-37,38-25-18-14-19-26-38)39-27-20-15-21-28-39/h13-21,23-32,41H,2-12,22,33-35H2,1H3,(H,45,46,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50206910

((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C38H55N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26-44-36(30-43-29-34-20-18-33(28-39)19-21-34)31-45-35-24-22-32(23-25-35)27-37-40-38(42)46-41-37/h18-25,36H,2-17,26-27,29-31H2,1H3,(H,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group X PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50206911

((+/+)-1-O-decyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCCCCCCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H60N2O5/c1-3-5-7-9-11-13-14-15-16-18-20-22-28-40-33(29-39-27-21-19-17-12-10-8-6-4-2)30-41-32-25-23-31(24-26-32)34-36-35(38)42-37-34/h23-26,33H,3-22,27-30H2,1-2H3,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group X PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50206910

((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C38H55N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26-44-36(30-43-29-34-20-18-33(28-39)19-21-34)31-45-35-24-22-32(23-25-35)27-37-40-38(42)46-41-37/h18-25,36H,2-17,26-27,29-31H2,1H3,(H,40,41,42) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206911

((+/+)-1-O-decyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCCCCCCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H60N2O5/c1-3-5-7-9-11-13-14-15-16-18-20-22-28-40-33(29-39-27-21-19-17-12-10-8-6-4-2)30-41-32-25-23-31(24-26-32)34-36-35(38)42-37-34/h23-26,33H,3-22,27-30H2,1-2H3,(H,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206908
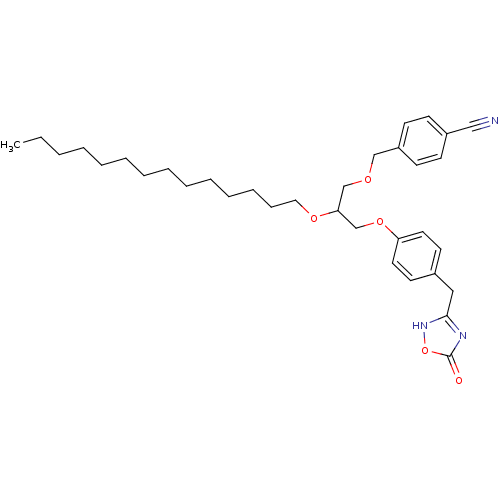
((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C34H47N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-40-32(26-39-25-30-16-14-29(24-35)15-17-30)27-41-31-20-18-28(19-21-31)23-33-36-34(38)42-37-33/h14-21,32H,2-13,22-23,25-27H2,1H3,(H,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206910

((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C38H55N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26-44-36(30-43-29-34-20-18-33(28-39)19-21-34)31-45-35-24-22-32(23-25-35)27-37-40-38(42)46-41-37/h18-25,36H,2-17,26-27,29-31H2,1H3,(H,40,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50206920

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(cc1)-c1nc(=O)o[nH]1)COC(c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C44H54N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-22-33-48-41(34-49-40-31-29-36(30-32-40)42-45-43(47)51-46-42)35-50-44(37-23-16-13-17-24-37,38-25-18-14-19-26-38)39-27-20-15-21-28-39/h13-21,23-32,41H,2-12,22,33-35H2,1H3,(H,45,46,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group X PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50206908

((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C34H47N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-40-32(26-39-25-30-16-14-29(24-35)15-17-30)27-41-31-20-18-28(19-21-31)23-33-36-34(38)42-37-33/h14-21,32H,2-13,22-23,25-27H2,1H3,(H,36,37,38) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50206913

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COC(c1ccccc1)c1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C38H50N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-19-28-42-35(29-43-34-26-24-33(25-27-34)37-39-38(41)45-40-37)30-44-36(31-20-15-13-16-21-31)32-22-17-14-18-23-32/h13-18,20-27,35-36H,2-12,19,28-30H2,1H3,(H,39,40,41) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206920

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(cc1)-c1nc(=O)o[nH]1)COC(c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C44H54N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-22-33-48-41(34-49-40-31-29-36(30-32-40)42-45-43(47)51-46-42)35-50-44(37-23-16-13-17-24-37,38-25-18-14-19-26-38)39-27-20-15-21-28-39/h13-21,23-32,41H,2-12,22,33-35H2,1H3,(H,45,46,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206918
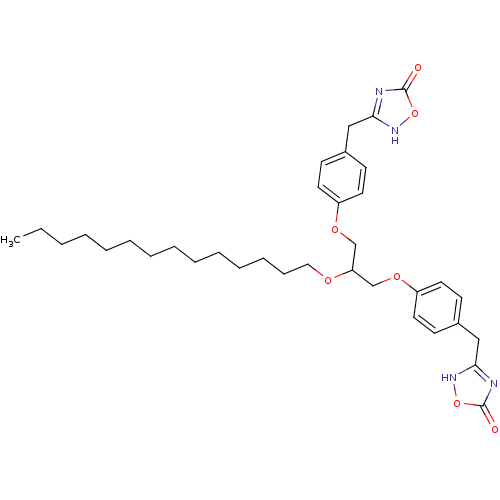
(1,3-O-di[4-(4,5-dihydro-5-oxo-4H-1,2,4-oxadiazol-3...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(Cc2nc(=O)o[nH]2)cc1)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C35H48N4O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-42-31(25-43-29-18-14-27(15-19-29)23-32-36-34(40)45-38-32)26-44-30-20-16-28(17-21-30)24-33-37-35(41)46-39-33/h14-21,31H,2-13,22-26H2,1H3,(H,36,38,40)(H,37,39,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206917

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COCc1cccc(OC)c1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C33H48N2O6/c1-3-4-5-6-7-8-9-10-11-12-13-14-22-39-31(25-38-24-27-16-15-17-30(23-27)37-2)26-40-29-20-18-28(19-21-29)32-34-33(36)41-35-32/h15-21,23,31H,3-14,22,24-26H2,1-2H3,(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206912

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H52N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-17-27-40-33(28-39-26-18-16-21-30-19-14-13-15-20-30)29-41-32-24-22-31(23-25-32)34-36-35(38)42-37-34/h13-15,19-20,22-25,33H,2-12,16-18,21,26-29H2,1H3,(H,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206914

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCOCC(COc1ccc(Cc2nc(=O)o[nH]2)cc1)OCCCCCCCCC Show InChI InChI=1S/C30H50N2O5/c1-3-5-7-9-11-13-15-21-34-24-28(35-22-16-14-12-10-8-6-4-2)25-36-27-19-17-26(18-20-27)23-29-31-30(33)37-32-29/h17-20,28H,3-16,21-25H2,1-2H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206916
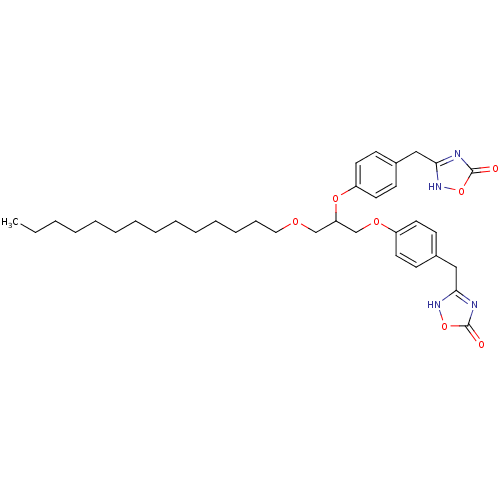
((+/-)-2,3-O-di[4-(4,5-dihydro-5-oxo-4H-1,2,4-oxadi...)Show SMILES CCCCCCCCCCCCCCOCC(COc1ccc(Cc2nc(=O)o[nH]2)cc1)Oc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C35H48N4O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-42-25-31(44-30-20-16-28(17-21-30)24-33-37-35(41)46-39-33)26-43-29-18-14-27(15-19-29)23-32-36-34(40)45-38-32/h14-21,31H,2-13,22-26H2,1H3,(H,36,38,40)(H,37,39,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50055371

((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IB PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM23770
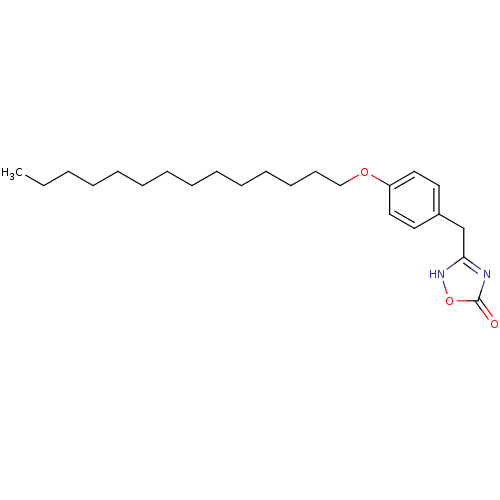
(3-{[4-(tetradecyloxy)phenyl]methyl}-4,5-dihydro-1,...)Show InChI InChI=1S/C23H36N2O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-18-27-21-16-14-20(15-17-21)19-22-24-23(26)28-25-22/h14-17H,2-13,18-19H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055371

((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group X PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206913

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COC(c1ccccc1)c1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C38H50N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-19-28-42-35(29-43-34-26-24-33(25-27-34)37-39-38(41)45-40-37)30-44-36(31-20-15-13-16-21-31)32-22-17-14-18-23-32/h13-18,20-27,35-36H,2-12,19,28-30H2,1H3,(H,39,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50206908

((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C34H47N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-40-32(26-39-25-30-16-14-29(24-35)15-17-30)27-41-31-20-18-28(19-21-31)23-33-36-34(38)42-37-33/h14-21,32H,2-13,22-23,25-27H2,1H3,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group X PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50206913

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COC(c1ccccc1)c1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C38H50N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-19-28-42-35(29-43-34-26-24-33(25-27-34)37-39-38(41)45-40-37)30-44-36(31-20-15-13-16-21-31)32-22-17-14-18-23-32/h13-18,20-27,35-36H,2-12,19,28-30H2,1H3,(H,39,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group X PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206913

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COC(c1ccccc1)c1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C38H50N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-19-28-42-35(29-43-34-26-24-33(25-27-34)37-39-38(41)45-40-37)30-44-36(31-20-15-13-16-21-31)32-22-17-14-18-23-32/h13-18,20-27,35-36H,2-12,19,28-30H2,1H3,(H,39,40,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50206920

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(cc1)-c1nc(=O)o[nH]1)COC(c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C44H54N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-22-33-48-41(34-49-40-31-29-36(30-32-40)42-45-43(47)51-46-42)35-50-44(37-23-16-13-17-24-37,38-25-18-14-19-26-38)39-27-20-15-21-28-39/h13-21,23-32,41H,2-12,22,33-35H2,1H3,(H,45,46,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IB PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206908

((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C34H47N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-40-32(26-39-25-30-16-14-29(24-35)15-17-30)27-41-31-20-18-28(19-21-31)23-33-36-34(38)42-37-33/h14-21,32H,2-13,22-23,25-27H2,1H3,(H,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50206908

((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C34H47N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-40-32(26-39-25-30-16-14-29(24-35)15-17-30)27-41-31-20-18-28(19-21-31)23-33-36-34(38)42-37-33/h14-21,32H,2-13,22-23,25-27H2,1H3,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IB PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM23771

((3-{[1-benzyl-3-(carbamoylmethyl)-2-ethyl-1H-indol...)Show SMILES CCc1c(CC(N)=O)c2cc(OCCCP(O)(O)=O)ccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H27N2O5P/c1-2-20-19(14-22(23)25)18-13-17(29-11-6-12-30(26,27)28)9-10-21(18)24(20)15-16-7-4-3-5-8-16/h3-5,7-10,13H,2,6,11-12,14-15H2,1H3,(H2,23,25)(H2,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206906
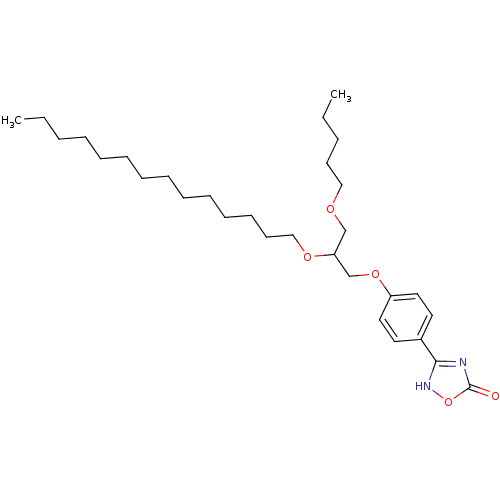
((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C30H50N2O5/c1-3-5-7-8-9-10-11-12-13-14-15-17-23-35-28(24-34-22-16-6-4-2)25-36-27-20-18-26(19-21-27)29-31-30(33)37-32-29/h18-21,28H,3-17,22-25H2,1-2H3,(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50206910

((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C38H55N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26-44-36(30-43-29-34-20-18-33(28-39)19-21-34)31-45-35-24-22-32(23-25-35)27-37-40-38(42)46-41-37/h18-25,36H,2-17,26-27,29-31H2,1H3,(H,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IB PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206908

((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C34H47N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-40-32(26-39-25-30-16-14-29(24-35)15-17-30)27-41-31-20-18-28(19-21-31)23-33-36-34(38)42-37-33/h14-21,32H,2-13,22-23,25-27H2,1H3,(H,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50206911

((+/+)-1-O-decyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCCCCCCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H60N2O5/c1-3-5-7-9-11-13-14-15-16-18-20-22-28-40-33(29-39-27-21-19-17-12-10-8-6-4-2)30-41-32-25-23-31(24-26-32)34-36-35(38)42-37-34/h23-26,33H,3-22,27-30H2,1-2H3,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IB PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206924

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCOCC(COc1ccc(Cc2nc(=O)o[nH]2)cc1)OCCCCCCC Show InChI InChI=1S/C26H42N2O5/c1-3-5-7-9-11-17-30-20-24(31-18-12-10-8-6-4-2)21-32-23-15-13-22(14-16-23)19-25-27-26(29)33-28-25/h13-16,24H,3-12,17-21H2,1-2H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50206912

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H52N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-17-27-40-33(28-39-26-18-16-21-30-19-14-13-15-20-30)29-41-32-24-22-31(23-25-32)34-36-35(38)42-37-34/h13-15,19-20,22-25,33H,2-12,16-18,21,26-29H2,1H3,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group X PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50206904

((+/-)-1-O-benzyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C32H46N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-16-23-37-30(25-36-24-27-17-14-13-15-18-27)26-38-29-21-19-28(20-22-29)31-33-32(35)39-34-31/h13-15,17-22,30H,2-12,16,23-26H2,1H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group X PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206904

((+/-)-1-O-benzyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C32H46N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-16-23-37-30(25-36-24-27-17-14-13-15-18-27)26-38-29-21-19-28(20-22-29)31-33-32(35)39-34-31/h13-15,17-22,30H,2-12,16,23-26H2,1H3,(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50206912

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H52N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-17-27-40-33(28-39-26-18-16-21-30-19-14-13-15-20-30)29-41-32-24-22-31(23-25-32)34-36-35(38)42-37-34/h13-15,19-20,22-25,33H,2-12,16-18,21,26-29H2,1H3,(H,36,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IB PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50206913

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COC(c1ccccc1)c1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C38H50N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-19-28-42-35(29-43-34-26-24-33(25-27-34)37-39-38(41)45-40-37)30-44-36(31-20-15-13-16-21-31)32-22-17-14-18-23-32/h13-18,20-27,35-36H,2-12,19,28-30H2,1H3,(H,39,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IB PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206923

((+/-)-2,3-O-di[4-(4,5-dihydro-5-oxo-4H-1,2,4-oxadi...)Show SMILES CCCCCCCCCCCCCCOCC(COc1ccc(cc1)-c1nc(=O)o[nH]1)Oc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C33H44N4O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-40-23-29(42-28-20-16-26(17-21-28)31-35-33(39)44-37-31)24-41-27-18-14-25(15-19-27)30-34-32(38)43-36-30/h14-21,29H,2-13,22-24H2,1H3,(H,34,36,38)(H,35,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50206912

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H52N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-17-27-40-33(28-39-26-18-16-21-30-19-14-13-15-20-30)29-41-32-24-22-31(23-25-32)34-36-35(38)42-37-34/h13-15,19-20,22-25,33H,2-12,16-18,21,26-29H2,1H3,(H,36,37,38) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206904

((+/-)-1-O-benzyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C32H46N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-16-23-37-30(25-36-24-27-17-14-13-15-18-27)26-38-29-21-19-28(20-22-29)31-33-32(35)39-34-31/h13-15,17-22,30H,2-12,16,23-26H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206912

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H52N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-17-27-40-33(28-39-26-18-16-21-30-19-14-13-15-20-30)29-41-32-24-22-31(23-25-32)34-36-35(38)42-37-34/h13-15,19-20,22-25,33H,2-12,16-18,21,26-29H2,1H3,(H,36,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206915
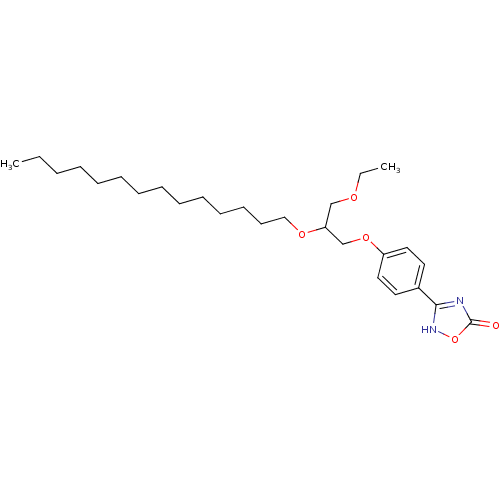
((+/-)-1-O-ethyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...)Show SMILES CCCCCCCCCCCCCCOC(COCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C27H44N2O5/c1-3-5-6-7-8-9-10-11-12-13-14-15-20-32-25(21-31-4-2)22-33-24-18-16-23(17-19-24)26-28-27(30)34-29-26/h16-19,25H,3-15,20-22H2,1-2H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206909
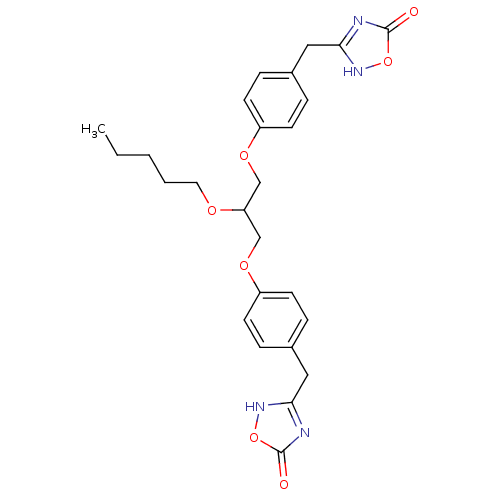
(1,3-O-di[4-(4,5-dihydro-5-oxo-4H-1,2,4-oxadiazol-3...)Show SMILES CCCCCOC(COc1ccc(Cc2nc(=O)o[nH]2)cc1)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C26H30N4O7/c1-2-3-4-13-33-22(16-34-20-9-5-18(6-10-20)14-23-27-25(31)36-29-23)17-35-21-11-7-19(8-12-21)15-24-28-26(32)37-30-24/h5-12,22H,2-4,13-17H2,1H3,(H,27,29,31)(H,28,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206910

((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C38H55N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26-44-36(30-43-29-34-20-18-33(28-39)19-21-34)31-45-35-24-22-32(23-25-35)27-37-40-38(42)46-41-37/h18-25,36H,2-17,26-27,29-31H2,1H3,(H,40,41,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206917

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COCc1cccc(OC)c1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C33H48N2O6/c1-3-4-5-6-7-8-9-10-11-12-13-14-22-39-31(25-38-24-27-16-15-17-30(23-27)37-2)26-40-29-20-18-28(19-21-29)32-34-33(36)41-35-32/h15-21,23,31H,3-14,22,24-26H2,1-2H3,(H,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206918

(1,3-O-di[4-(4,5-dihydro-5-oxo-4H-1,2,4-oxadiazol-3...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(Cc2nc(=O)o[nH]2)cc1)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C35H48N4O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-42-31(25-43-29-18-14-27(15-19-29)23-32-36-34(40)45-38-32)26-44-30-20-16-28(17-21-30)24-33-37-35(41)46-39-33/h14-21,31H,2-13,22-26H2,1H3,(H,36,38,40)(H,37,39,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206915

((+/-)-1-O-ethyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...)Show SMILES CCCCCCCCCCCCCCOC(COCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C27H44N2O5/c1-3-5-6-7-8-9-10-11-12-13-14-15-20-32-25(21-31-4-2)22-33-24-18-16-23(17-19-24)26-28-27(30)34-29-26/h16-19,25H,3-15,20-22H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206921
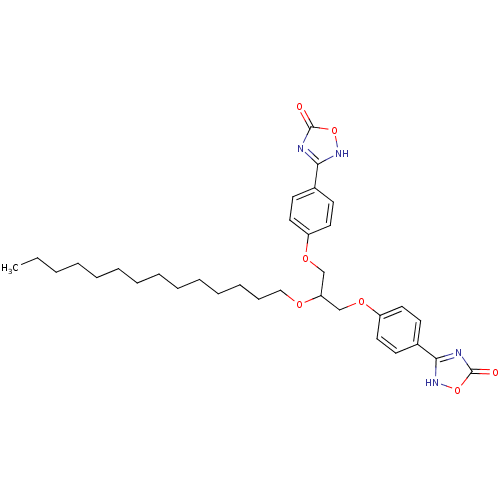
(1,3-O-di[4-(4,5-dihydro-5-oxo-4H-1,2,4-oxadiazol-3...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(cc1)-c1nc(=O)o[nH]1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C33H44N4O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-40-29(23-41-27-18-14-25(15-19-27)30-34-32(38)43-36-30)24-42-28-20-16-26(17-21-28)31-35-33(39)44-37-31/h14-21,29H,2-13,22-24H2,1H3,(H,34,36,38)(H,35,37,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206920

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(cc1)-c1nc(=O)o[nH]1)COC(c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C44H54N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-22-33-48-41(34-49-40-31-29-36(30-32-40)42-45-43(47)51-46-42)35-50-44(37-23-16-13-17-24-37,38-25-18-14-19-26-38)39-27-20-15-21-28-39/h13-21,23-32,41H,2-12,22,33-35H2,1H3,(H,45,46,47) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206905

((S)-3-(4-(2-(tetradecyloxy)-3-(trityloxy)propoxy)p...)Show SMILES CCCCCCCCCCCCCCO[C@@H](COc1ccc(cc1)-c1nc(=O)o[nH]1)COC(c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C44H54N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-22-33-48-41(34-49-40-31-29-36(30-32-40)42-45-43(47)51-46-42)35-50-44(37-23-16-13-17-24-37,38-25-18-14-19-26-38)39-27-20-15-21-28-39/h13-21,23-32,41H,2-12,22,33-35H2,1H3,(H,45,46,47)/t41-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206922
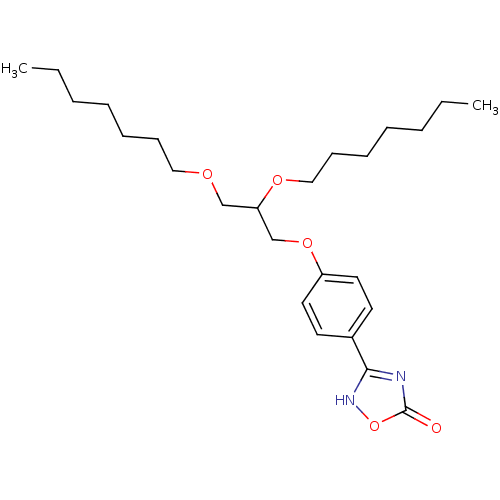
((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCOCC(COc1ccc(cc1)-c1nc(=O)o[nH]1)OCCCCCCC Show InChI InChI=1S/C25H40N2O5/c1-3-5-7-9-11-17-29-19-23(30-18-12-10-8-6-4-2)20-31-22-15-13-21(14-16-22)24-26-25(28)32-27-24/h13-16,23H,3-12,17-20H2,1-2H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206919
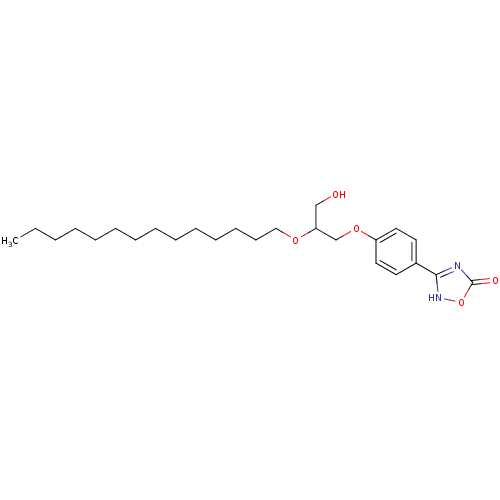
((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(CO)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C25H40N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-18-30-23(19-28)20-31-22-16-14-21(15-17-22)24-26-25(29)32-27-24/h14-17,23,28H,2-13,18-20H2,1H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206916

((+/-)-2,3-O-di[4-(4,5-dihydro-5-oxo-4H-1,2,4-oxadi...)Show SMILES CCCCCCCCCCCCCCOCC(COc1ccc(Cc2nc(=O)o[nH]2)cc1)Oc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C35H48N4O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-42-25-31(44-30-20-16-28(17-21-30)24-33-37-35(41)46-39-33)26-43-29-18-14-27(15-19-29)23-32-36-34(40)45-38-32/h14-21,31H,2-13,22-26H2,1H3,(H,36,38,40)(H,37,39,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206923

((+/-)-2,3-O-di[4-(4,5-dihydro-5-oxo-4H-1,2,4-oxadi...)Show SMILES CCCCCCCCCCCCCCOCC(COc1ccc(cc1)-c1nc(=O)o[nH]1)Oc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C33H44N4O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-40-23-29(42-28-20-16-26(17-21-28)31-35-33(39)44-37-31)24-41-27-18-14-25(15-19-27)30-34-32(38)43-36-30/h14-21,29H,2-13,22-24H2,1H3,(H,34,36,38)(H,35,37,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206907

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES O=c1nc([nH]o1)-c1ccc(OCC(COCCCCc2ccccc2)OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C31H36N2O5/c34-31-32-30(33-38-31)27-17-19-28(20-18-27)37-24-29(36-22-10-8-16-26-13-5-2-6-14-26)23-35-21-9-7-15-25-11-3-1-4-12-25/h1-6,11-14,17-20,29H,7-10,15-16,21-24H2,(H,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206919

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(CO)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C25H40N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-18-30-23(19-28)20-31-22-16-14-21(15-17-22)24-26-25(29)32-27-24/h14-17,23,28H,2-13,18-20H2,1H3,(H,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50206904

((+/-)-1-O-benzyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C32H46N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-16-23-37-30(25-36-24-27-17-14-13-15-18-27)26-38-29-21-19-28(20-22-29)31-33-32(35)39-34-31/h13-15,17-22,30H,2-12,16,23-26H2,1H3,(H,33,34,35) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2
(Homo sapiens (Human)) | BDBM50206904

((+/-)-1-O-benzyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C32H46N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-16-23-37-30(25-36-24-27-17-14-13-15-18-27)26-38-29-21-19-28(20-22-29)31-33-32(35)39-34-31/h13-15,17-22,30H,2-12,16,23-26H2,1H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IB PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206906

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C30H50N2O5/c1-3-5-7-8-9-10-11-12-13-14-15-17-23-35-28(24-34-22-16-6-4-2)25-36-27-20-18-26(19-21-27)29-31-30(33)37-32-29/h18-21,28H,3-17,22-25H2,1-2H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206921

(1,3-O-di[4-(4,5-dihydro-5-oxo-4H-1,2,4-oxadiazol-3...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(cc1)-c1nc(=O)o[nH]1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C33H44N4O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-22-40-29(23-41-27-18-14-25(15-19-27)30-34-32(38)43-36-30)24-42-28-20-16-26(17-21-28)31-35-33(39)44-37-31/h14-21,29H,2-13,22-24H2,1H3,(H,34,36,38)(H,35,37,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206924

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCOCC(COc1ccc(Cc2nc(=O)o[nH]2)cc1)OCCCCCCC Show InChI InChI=1S/C26H42N2O5/c1-3-5-7-9-11-17-30-20-24(31-18-12-10-8-6-4-2)21-32-23-15-13-22(14-16-23)19-25-27-26(29)33-28-25/h13-16,24H,3-12,17-21H2,1-2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM23770

(3-{[4-(tetradecyloxy)phenyl]methyl}-4,5-dihydro-1,...)Show InChI InChI=1S/C23H36N2O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-18-27-21-16-14-20(15-17-21)19-22-24-23(26)28-25-22/h14-17H,2-13,18-19H2,1H3,(H,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206911

((+/+)-1-O-decyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCCCCCCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H60N2O5/c1-3-5-7-9-11-13-14-15-16-18-20-22-28-40-33(29-39-27-21-19-17-12-10-8-6-4-2)30-41-32-25-23-31(24-26-32)34-36-35(38)42-37-34/h23-26,33H,3-22,27-30H2,1-2H3,(H,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50206904

((+/-)-1-O-benzyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4...)Show SMILES CCCCCCCCCCCCCCOC(COCc1ccccc1)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C32H46N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-16-23-37-30(25-36-24-27-17-14-13-15-18-27)26-38-29-21-19-28(20-22-29)31-33-32(35)39-34-31/h13-15,17-22,30H,2-12,16,23-26H2,1H3,(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group IIA PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206914

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCOCC(COc1ccc(Cc2nc(=O)o[nH]2)cc1)OCCCCCCCCC Show InChI InChI=1S/C30H50N2O5/c1-3-5-7-9-11-13-15-21-34-24-28(35-22-16-14-12-10-8-6-4-2)25-36-27-19-17-26(18-20-27)23-29-31-30(33)37-32-29/h17-20,28H,3-16,21-25H2,1-2H3,(H,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206907

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES O=c1nc([nH]o1)-c1ccc(OCC(COCCCCc2ccccc2)OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C31H36N2O5/c34-31-32-30(33-38-31)27-17-19-28(20-18-27)37-24-29(36-22-10-8-16-26-13-5-2-6-14-26)23-35-21-9-7-15-25-11-3-1-4-12-25/h1-6,11-14,17-20,29H,7-10,15-16,21-24H2,(H,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206909

(1,3-O-di[4-(4,5-dihydro-5-oxo-4H-1,2,4-oxadiazol-3...)Show SMILES CCCCCOC(COc1ccc(Cc2nc(=O)o[nH]2)cc1)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C26H30N4O7/c1-2-3-4-13-33-22(16-34-20-9-5-18(6-10-20)14-23-27-25(31)36-29-23)17-35-21-11-7-19(8-12-21)15-24-28-26(32)37-30-24/h5-12,22H,2-4,13-17H2,1H3,(H,27,29,31)(H,28,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50206922

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCOCC(COc1ccc(cc1)-c1nc(=O)o[nH]1)OCCCCCCC Show InChI InChI=1S/C25H40N2O5/c1-3-5-7-9-11-17-29-19-23(30-18-12-10-8-6-4-2)20-31-22-15-13-21(14-16-22)24-26-25(28)32-27-24/h13-16,23H,3-12,17-20H2,1-2H3,(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of pig group IB PLA2 by fluorimetric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data