 Found 26 hits of Enzyme Inhibition Constant Data
Found 26 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215008
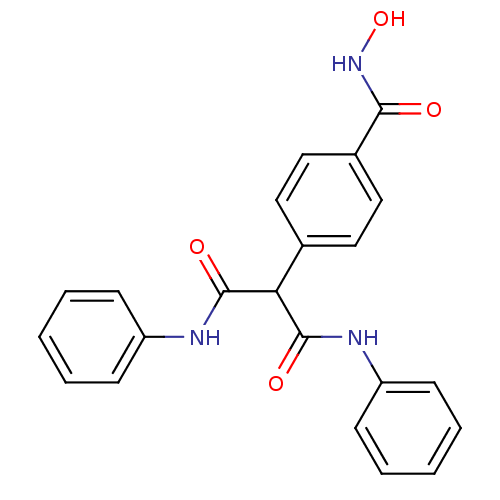
(4-(1,3-dioxo-1,3-bis(phenylamino)propan-2-yl)-N-hy...)Show SMILES ONC(=O)c1ccc(cc1)C(C(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C22H19N3O4/c26-20(25-29)16-13-11-15(12-14-16)19(21(27)23-17-7-3-1-4-8-17)22(28)24-18-9-5-2-6-10-18/h1-14,19,29H,(H,23,27)(H,24,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215018

(4-(1,3-bis(2,3-dihydrobenzo[b][1,4]dioxin-6-ylamin...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1ccc2OCCOc2c1)C(=O)Nc1ccc2OCCOc2c1 Show InChI InChI=1S/C32H28N4O7/c33-23-3-1-2-4-24(23)36-30(37)20-7-5-19(6-8-20)29(31(38)34-21-9-11-25-27(17-21)42-15-13-40-25)32(39)35-22-10-12-26-28(18-22)43-16-14-41-26/h1-12,17-18,29H,13-16,33H2,(H,34,38)(H,35,39)(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215015
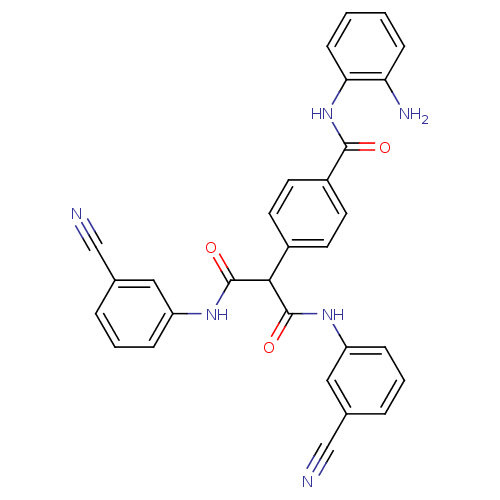
(4-(1,3-bis(3-cyanophenylamino)-1,3-dioxopropan-2-y...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1cccc(c1)C#N)C(=O)Nc1cccc(c1)C#N Show InChI InChI=1S/C30H22N6O3/c31-17-19-5-3-7-23(15-19)34-29(38)27(30(39)35-24-8-4-6-20(16-24)18-32)21-11-13-22(14-12-21)28(37)36-26-10-2-1-9-25(26)33/h1-16,27H,33H2,(H,34,38)(H,35,39)(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215016

(4-(1,3-bis(3-methoxyphenylamino)-1,3-dioxopropan-2...)Show SMILES COc1cccc(NC(=O)C(C(=O)Nc2cccc(OC)c2)c2ccc(cc2)C(=O)Nc2ccccc2N)c1 Show InChI InChI=1S/C30H28N4O5/c1-38-23-9-5-7-21(17-23)32-29(36)27(30(37)33-22-8-6-10-24(18-22)39-2)19-13-15-20(16-14-19)28(35)34-26-12-4-3-11-25(26)31/h3-18,27H,31H2,1-2H3,(H,32,36)(H,33,37)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215010

(CHEMBL251144 | N-(2-aminophenyl)-4-(1,3-dioxo-1,3-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C28H24N4O3/c29-23-13-7-8-14-24(23)32-26(33)20-17-15-19(16-18-20)25(27(34)30-21-9-3-1-4-10-21)28(35)31-22-11-5-2-6-12-22/h1-18,25H,29H2,(H,30,34)(H,31,35)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215014
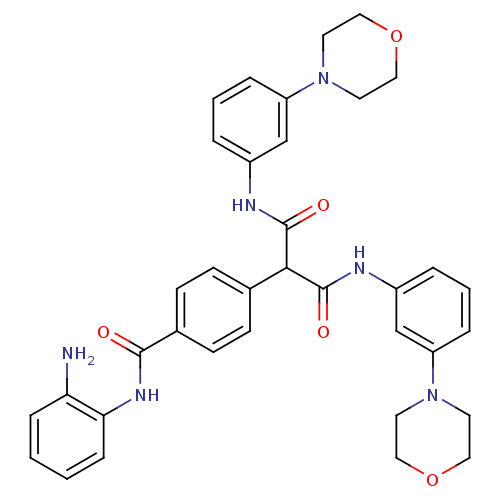
(4-(1,3-bis(3-morpholinophenylamino)-1,3-dioxopropa...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1cccc(c1)N1CCOCC1)C(=O)Nc1cccc(c1)N1CCOCC1 Show InChI InChI=1S/C36H38N6O5/c37-31-9-1-2-10-32(31)40-34(43)26-13-11-25(12-14-26)33(35(44)38-27-5-3-7-29(23-27)41-15-19-46-20-16-41)36(45)39-28-6-4-8-30(24-28)42-17-21-47-22-18-42/h1-14,23-24,33H,15-22,37H2,(H,38,44)(H,39,45)(H,40,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215013

(CHEMBL398950 | N-(2-aminophenyl)-4-(3-oxo-3-(pheny...)Show SMILES Nc1ccccc1NC(=O)c1ccc(CC(C(=O)Nc2ccccc2)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C29H26N4O3/c30-25-13-7-8-14-26(25)33-27(34)21-17-15-20(16-18-21)19-24(28(35)31-22-9-3-1-4-10-22)29(36)32-23-11-5-2-6-12-23/h1-18,24H,19,30H2,(H,31,35)(H,32,36)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215003

(4-(1,3-bis(2-methoxyphenylamino)-1,3-dioxopropan-2...)Show SMILES COc1ccccc1NC(=O)C(C(=O)Nc1ccccc1OC)c1ccc(cc1)C(=O)Nc1ccccc1N Show InChI InChI=1S/C30H28N4O5/c1-38-25-13-7-5-11-23(25)33-29(36)27(30(37)34-24-12-6-8-14-26(24)39-2)19-15-17-20(18-16-19)28(35)32-22-10-4-3-9-21(22)31/h3-18,27H,31H2,1-2H3,(H,32,35)(H,33,36)(H,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215012

(CHEMBL251756 | N-(2-aminophenyl)-4-(2-fluoro-1,3-d...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(F)(C(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C28H23FN4O3/c29-28(26(35)31-21-9-3-1-4-10-21,27(36)32-22-11-5-2-6-12-22)20-17-15-19(16-18-20)25(34)33-24-14-8-7-13-23(24)30/h1-18H,30H2,(H,31,35)(H,32,36)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215004
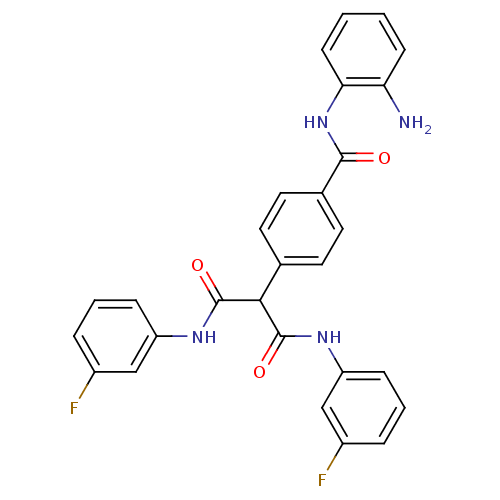
(4-(1,3-bis(3-fluorophenylamino)-1,3-dioxopropan-2-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1cccc(F)c1)C(=O)Nc1cccc(F)c1 Show InChI InChI=1S/C28H22F2N4O3/c29-19-5-3-7-21(15-19)32-27(36)25(28(37)33-22-8-4-6-20(30)16-22)17-11-13-18(14-12-17)26(35)34-24-10-2-1-9-23(24)31/h1-16,25H,31H2,(H,32,36)(H,33,37)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215005

(4-(1,3-bis(4-fluorophenylamino)-1,3-dioxopropan-2-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1ccc(F)cc1)C(=O)Nc1ccc(F)cc1 Show InChI InChI=1S/C28H22F2N4O3/c29-19-9-13-21(14-10-19)32-27(36)25(28(37)33-22-15-11-20(30)12-16-22)17-5-7-18(8-6-17)26(35)34-24-4-2-1-3-23(24)31/h1-16,25H,31H2,(H,32,36)(H,33,37)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215009
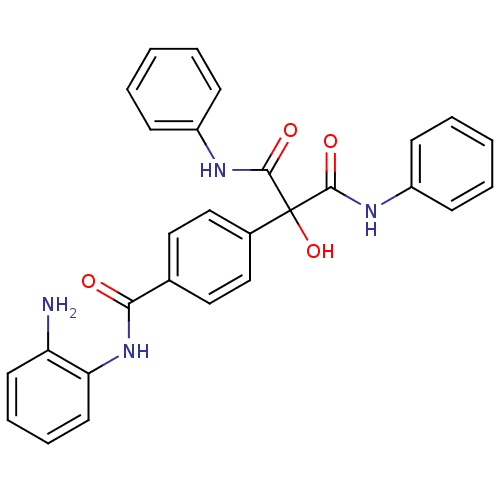
(CHEMBL251757 | N-(2-aminophenyl)-4-(2-hydroxy-1,3-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(O)(C(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C28H24N4O4/c29-23-13-7-8-14-24(23)32-25(33)19-15-17-20(18-16-19)28(36,26(34)30-21-9-3-1-4-10-21)27(35)31-22-11-5-2-6-12-22/h1-18,36H,29H2,(H,30,34)(H,31,35)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215011

(CHEMBL400921 | N-(2-aminophenyl)-4-(1,3-dioxo-1,3-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1cccc2cccnc12)C(=O)Nc1cccc2cccnc12 Show InChI InChI=1S/C34H26N6O3/c35-25-11-1-2-12-26(25)38-32(41)24-17-15-21(16-18-24)29(33(42)39-27-13-3-7-22-9-5-19-36-30(22)27)34(43)40-28-14-4-8-23-10-6-20-37-31(23)28/h1-20,29H,35H2,(H,38,41)(H,39,42)(H,40,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215017

(CHEMBL251544 | N-(2-aminophenyl)-4-(3-(methyl(phen...)Show SMILES CN(C(=O)C(C(=O)Nc1ccccc1)c1ccc(cc1)C(=O)Nc1ccccc1N)c1ccccc1 |w:4.14| Show InChI InChI=1S/C29H26N4O3/c1-33(23-12-6-3-7-13-23)29(36)26(28(35)31-22-10-4-2-5-11-22)20-16-18-21(19-17-20)27(34)32-25-15-9-8-14-24(25)30/h2-19,26H,30H2,1H3,(H,31,35)(H,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215021
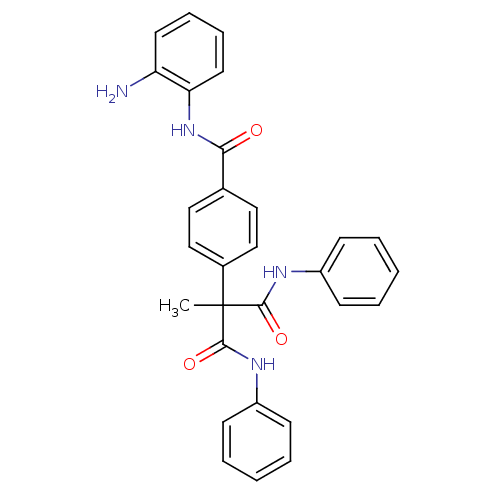
(CHEMBL248718 | N-(2-aminophenyl)-4-(2-methyl-1,3-d...)Show SMILES CC(C(=O)Nc1ccccc1)(C(=O)Nc1ccccc1)c1ccc(cc1)C(=O)Nc1ccccc1N Show InChI InChI=1S/C29H26N4O3/c1-29(27(35)31-22-10-4-2-5-11-22,28(36)32-23-12-6-3-7-13-23)21-18-16-20(17-19-21)26(34)33-25-15-9-8-14-24(25)30/h2-19H,30H2,1H3,(H,31,35)(H,32,36)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215002

(4-(1,3-bis(benzylamino)-1,3-dioxopropan-2-yl)-N-(2...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)NCc1ccccc1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C30H28N4O3/c31-25-13-7-8-14-26(25)34-28(35)24-17-15-23(16-18-24)27(29(36)32-19-21-9-3-1-4-10-21)30(37)33-20-22-11-5-2-6-12-22/h1-18,27H,19-20,31H2,(H,32,36)(H,33,37)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215007
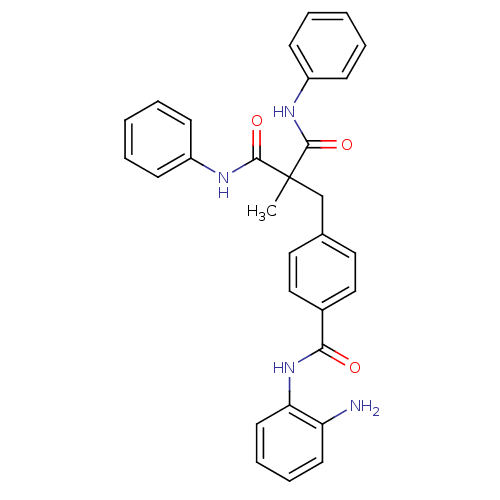
(CHEMBL248909 | N-(2-aminophenyl)-4-(2-methyl-3-oxo...)Show SMILES CC(Cc1ccc(cc1)C(=O)Nc1ccccc1N)(C(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C30H28N4O3/c1-30(28(36)32-23-10-4-2-5-11-23,29(37)33-24-12-6-3-7-13-24)20-21-16-18-22(19-17-21)27(35)34-26-15-9-8-14-25(26)31/h2-19H,20,31H2,1H3,(H,32,36)(H,33,37)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215006

(CHEMBL251546 | N-(2-aminophenyl)-4-(1,3-dioxo-1,3-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)NCCCc1ccccc1)C(=O)NCCCc1ccccc1 Show InChI InChI=1S/C34H36N4O3/c35-29-17-7-8-18-30(29)38-32(39)28-21-19-27(20-22-28)31(33(40)36-23-9-15-25-11-3-1-4-12-25)34(41)37-24-10-16-26-13-5-2-6-14-26/h1-8,11-14,17-22,31H,9-10,15-16,23-24,35H2,(H,36,40)(H,37,41)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 229 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50215010

(CHEMBL251144 | N-(2-aminophenyl)-4-(1,3-dioxo-1,3-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C28H24N4O3/c29-23-13-7-8-14-24(23)32-26(33)20-17-15-19(16-18-20)25(27(34)30-21-9-3-1-4-10-21)28(35)31-22-11-5-2-6-12-22/h1-18,25H,29H2,(H,30,34)(H,31,35)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 313 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC2 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50215010

(CHEMBL251144 | N-(2-aminophenyl)-4-(1,3-dioxo-1,3-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C28H24N4O3/c29-23-13-7-8-14-24(23)32-26(33)20-17-15-19(16-18-20)25(27(34)30-21-9-3-1-4-10-21)28(35)31-22-11-5-2-6-12-22/h1-18,25H,29H2,(H,30,34)(H,31,35)(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 697 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC3 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215019
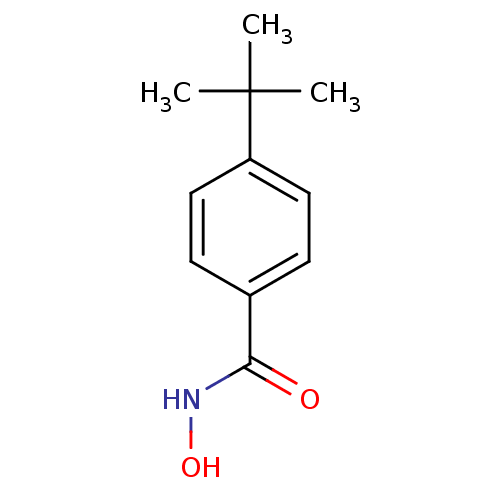
(4-tert-butyl-N-hydroxybenzamide | CHEMBL249284)Show InChI InChI=1S/C11H15NO2/c1-11(2,3)9-6-4-8(5-7-9)10(13)12-14/h4-7,14H,1-3H3,(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 715 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50215020

(4-(1,3-bis(methyl(phenyl)amino)-1,3-dioxopropan-2-...)Show SMILES CN(C(=O)C(C(=O)N(C)c1ccccc1)c1ccc(cc1)C(=O)Nc1ccccc1N)c1ccccc1 Show InChI InChI=1S/C30H28N4O3/c1-33(23-11-5-3-6-12-23)29(36)27(30(37)34(2)24-13-7-4-8-14-24)21-17-19-22(20-18-21)28(35)32-26-16-10-9-15-25(26)31/h3-20,27H,31H2,1-2H3,(H,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50215010

(CHEMBL251144 | N-(2-aminophenyl)-4-(1,3-dioxo-1,3-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C28H24N4O3/c29-23-13-7-8-14-24(23)32-26(33)20-17-15-19(16-18-20)25(27(34)30-21-9-3-1-4-10-21)28(35)31-22-11-5-2-6-12-22/h1-18,25H,29H2,(H,30,34)(H,31,35)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50215010

(CHEMBL251144 | N-(2-aminophenyl)-4-(1,3-dioxo-1,3-...)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(C(=O)Nc1ccccc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C28H24N4O3/c29-23-13-7-8-14-24(23)32-26(33)20-17-15-19(16-18-20)25(27(34)30-21-9-3-1-4-10-21)28(35)31-22-11-5-2-6-12-22/h1-18,25H,29H2,(H,30,34)(H,31,35)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50015184

(BENZHYDROXAMIC ACID | BENZOHYDROXAMATE | CHEMBL163...)Show InChI InChI=1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50099857
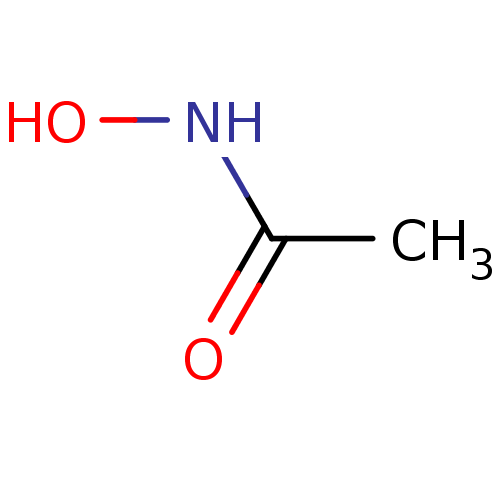
(ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...)Show InChI InChI=1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 expressed in domain of SMRT |
Bioorg Med Chem Lett 17: 4619-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.080
BindingDB Entry DOI: 10.7270/Q2WH2PPV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data