

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23079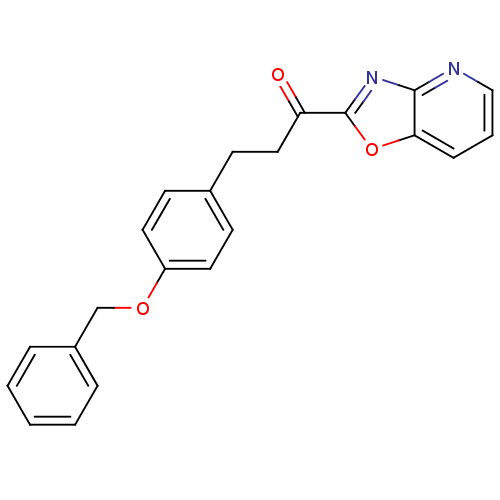 (3-[4-(benzyloxy)phenyl]-1-{pyrido[2,3-d][1,3]oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | -53.8 | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23069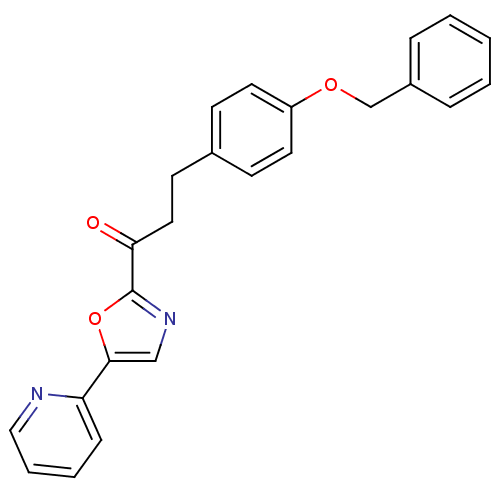 (3-[4-(benzyloxy)phenyl]-1-[5-(pyridin-2-yl)-1,3-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | -53.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23079 (3-[4-(benzyloxy)phenyl]-1-{pyrido[2,3-d][1,3]oxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23078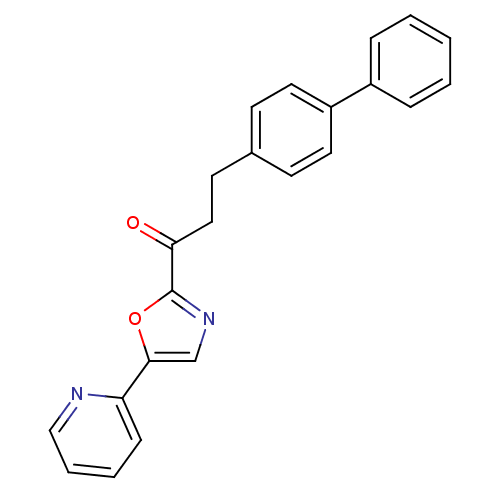 (3-(4-phenylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | -52.1 | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23061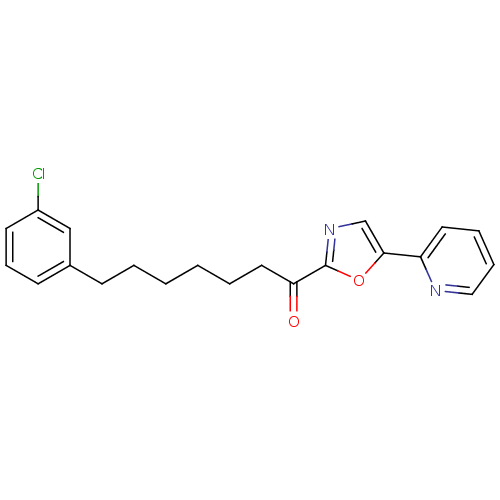 (7-(3-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | -51.6 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23063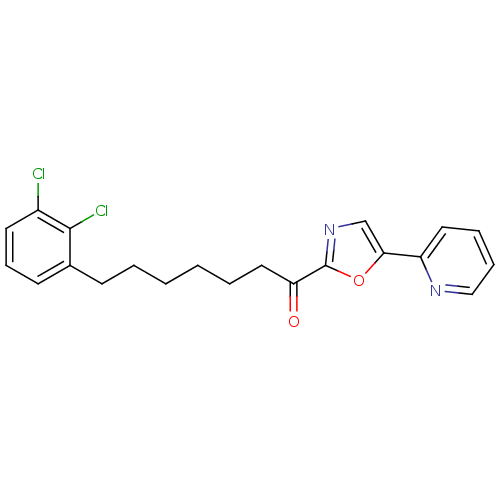 (7-(2,3-dichlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | -51.6 | 7 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23055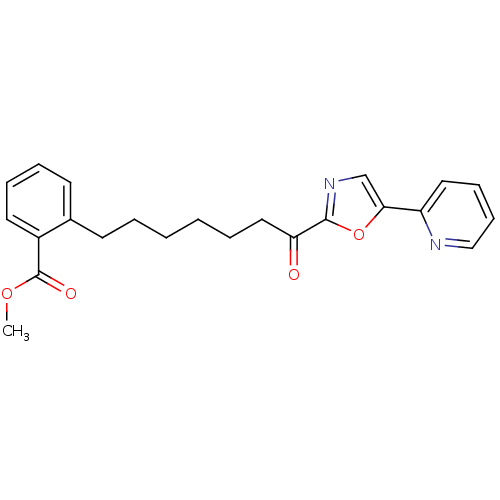 (alpha-ketooxazole, 5aa | methyl 2-{7-oxo-7-[5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -50.9 | 0.400 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23073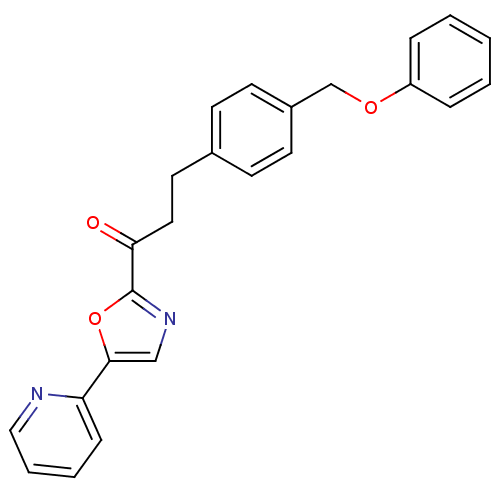 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -51.4 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23053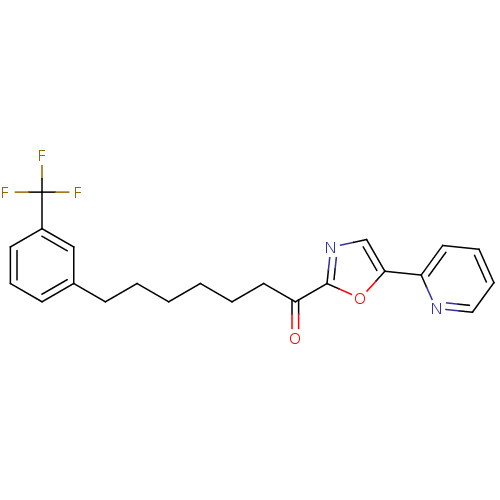 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[3-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -50.9 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23069 (3-[4-(benzyloxy)phenyl]-1-[5-(pyridin-2-yl)-1,3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | -50.7 | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23065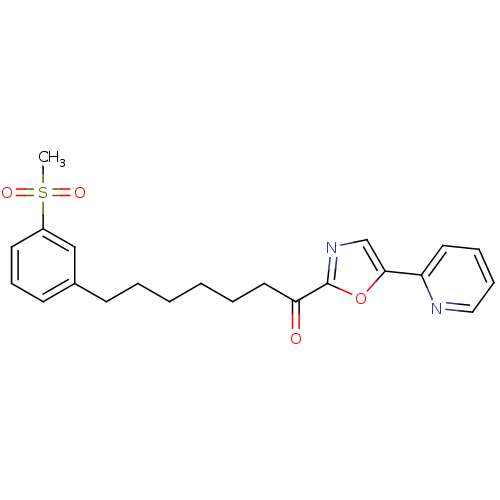 (7-(3-methanesulfonylphenyl)-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | -50.7 | 4 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23057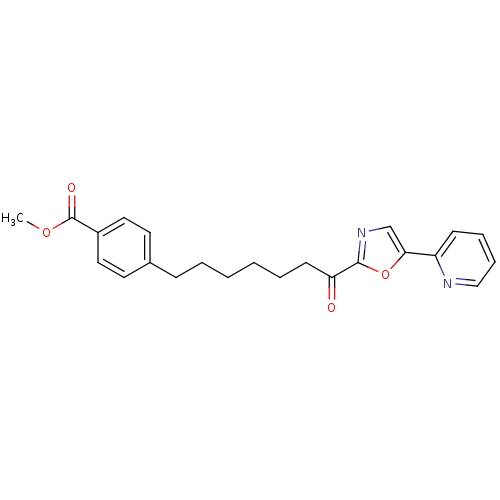 (alpha-ketooxazole, 5cc | methyl 4-{7-oxo-7-[5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | -49.9 | 0.240 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23045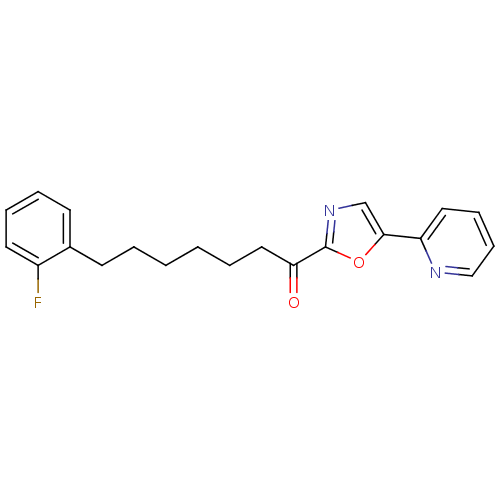 (7-(2-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | -49.6 | 30 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23056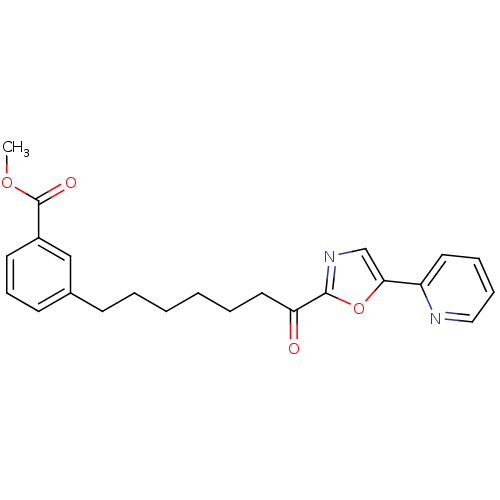 (alpha-ketooxazole, 5bb | methyl 3-{7-oxo-7-[5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | -49.3 | 0.400 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23060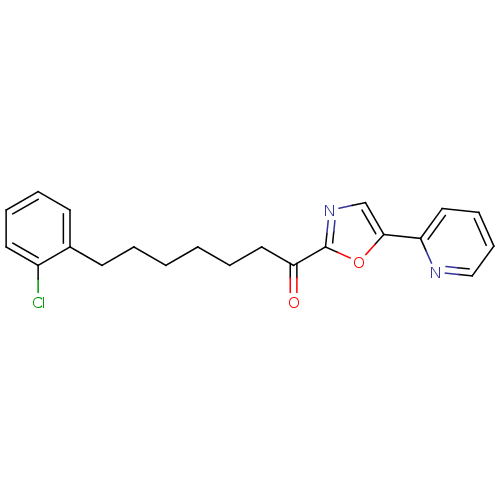 (7-(2-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | -49.8 | 20 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23075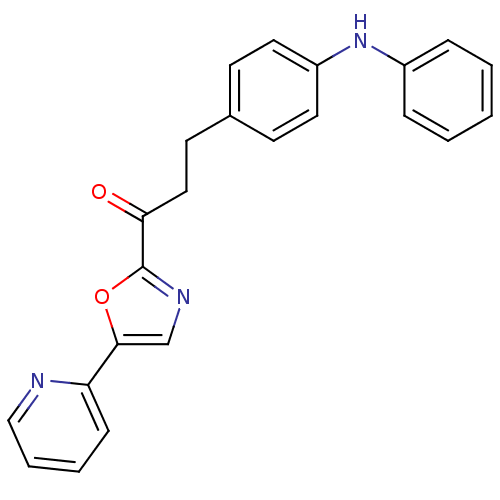 (3-[4-(phenylamino)phenyl]-1-[5-(pyridin-2-yl)-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | -49.7 | 5 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23077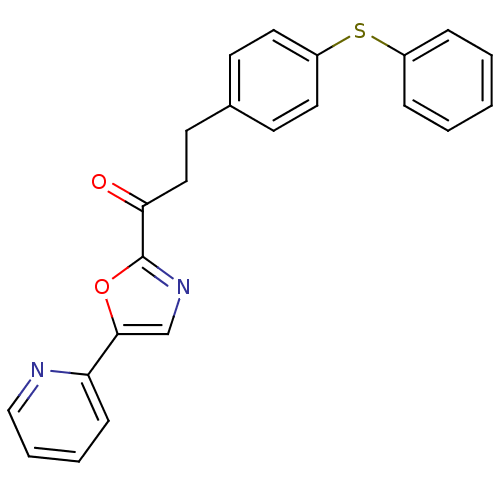 (3-[4-(phenylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | -49.4 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23046 (7-(3-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | -48.9 | 10 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23043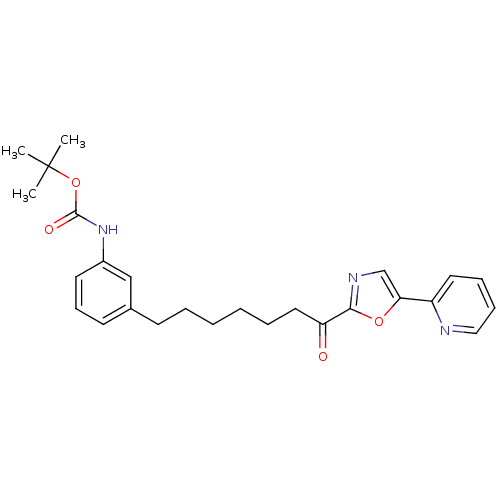 (alpha-ketooxazole, 5o | tert-butyl N-(3-{7-oxo-7-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40 | -48.7 | n/a | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23039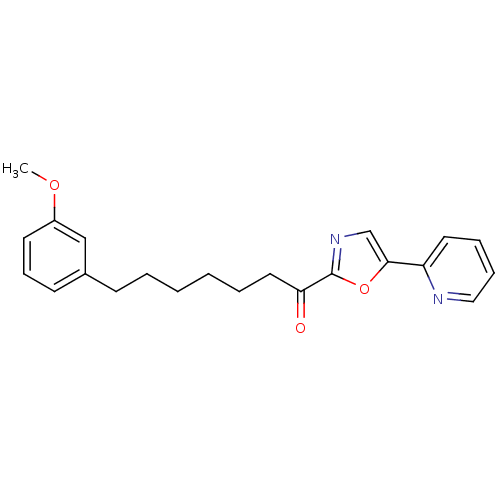 (7-(3-methoxyphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -48.6 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23050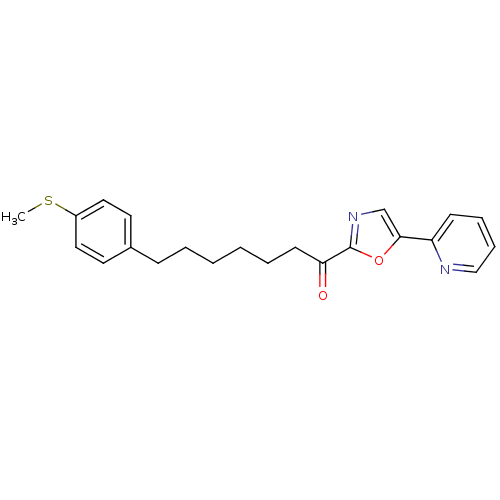 (7-[4-(methylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | -48.6 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23030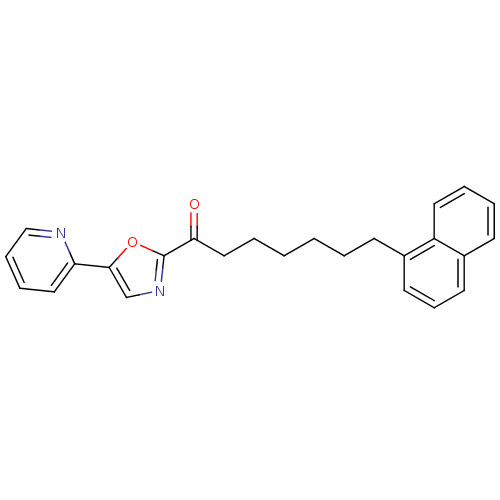 (7-(naphthalen-1-yl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | -48.5 | 10 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23062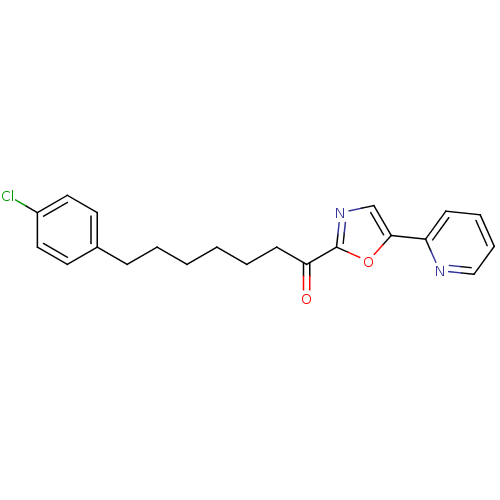 (7-(4-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | -48.9 | 30 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23037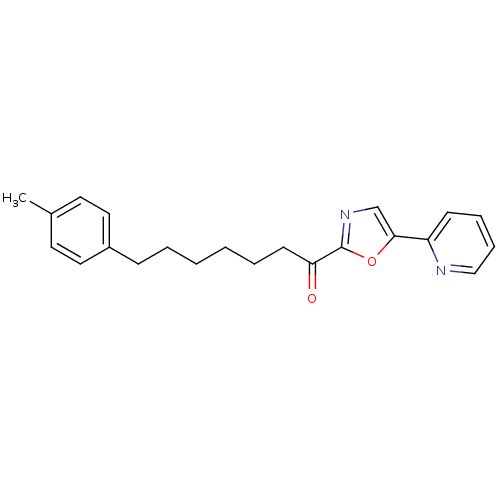 (7-(4-methylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | -48.3 | 40 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23078 (3-(4-phenylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | -48.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23042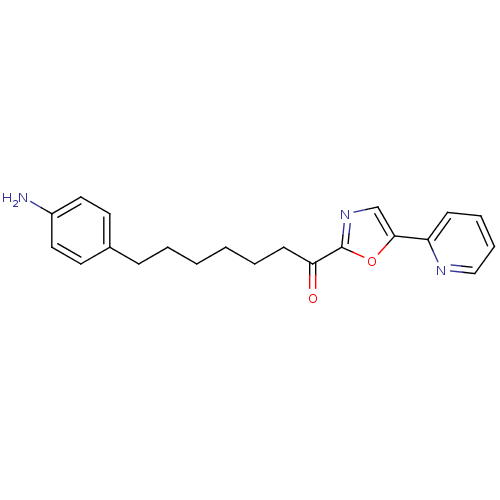 (7-(4-aminophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | 4 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23034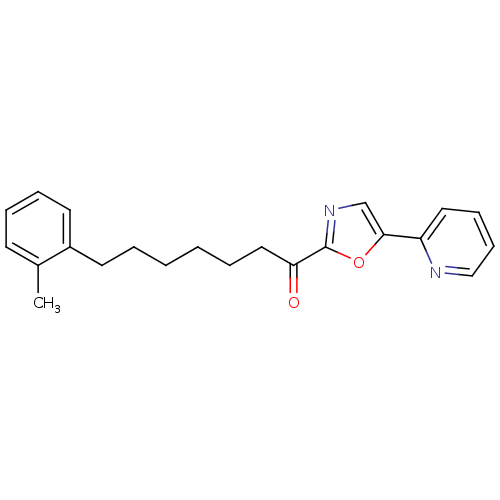 (7-(2-methylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | 30 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23117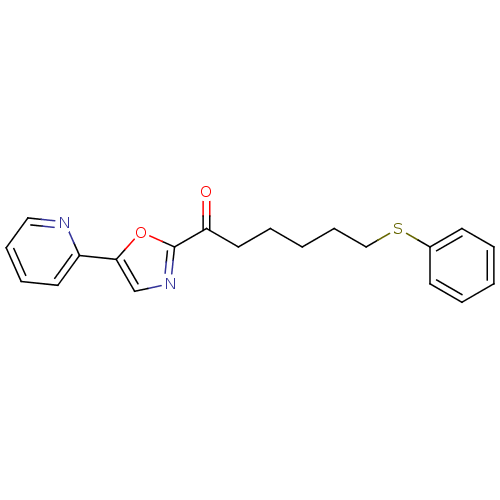 (6-(phenylsulfanyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23076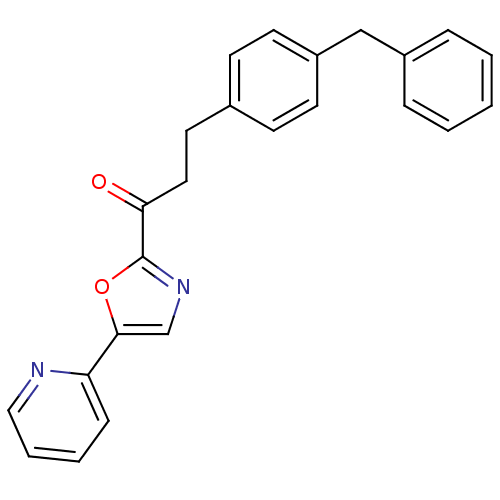 (3-(4-benzylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | -48.5 | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23047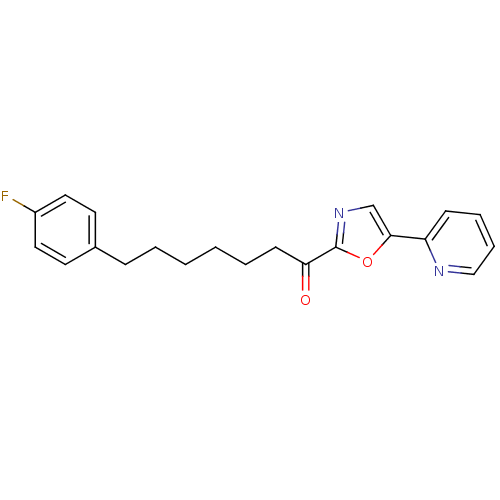 (7-(4-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | -48.0 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23087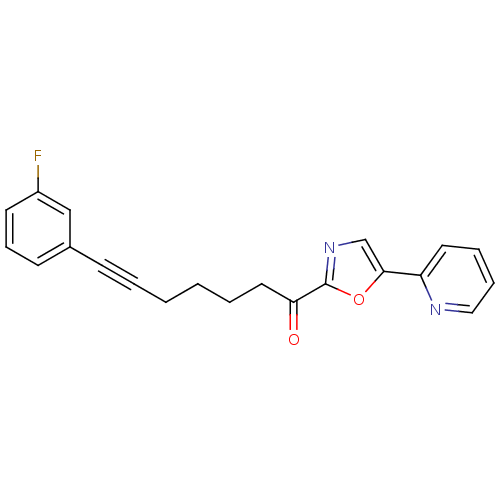 (7-(3-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.20 | -48.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23036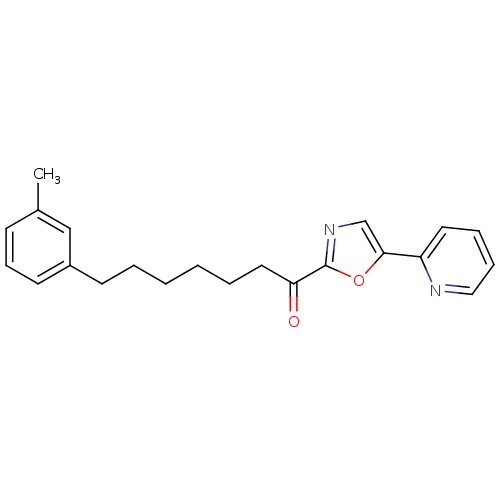 (7-(3-methylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | -47.9 | 30 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23048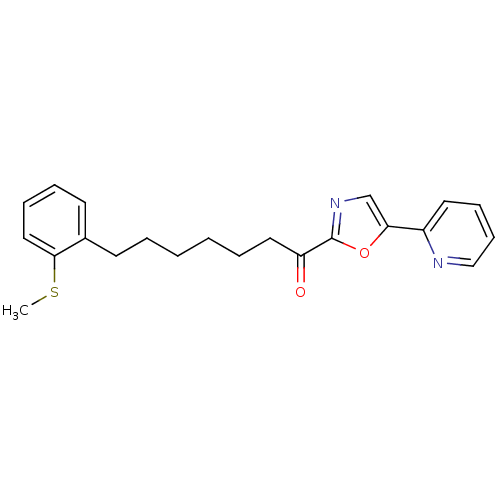 (7-[2-(methylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.30 | -47.9 | 30 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23074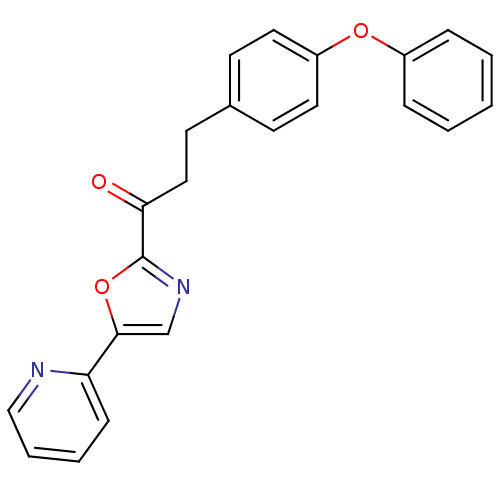 (3-(4-phenoxyphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | -48.3 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23054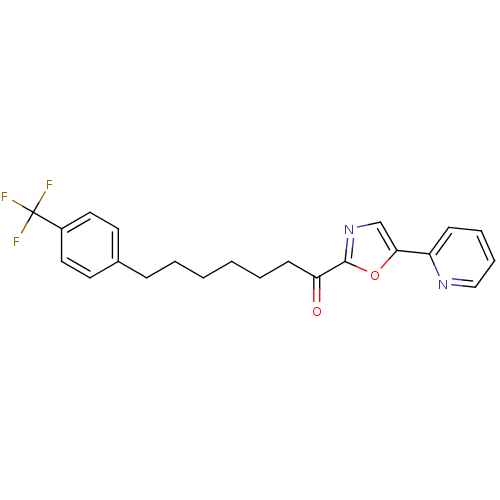 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[4-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.5 | 9 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23052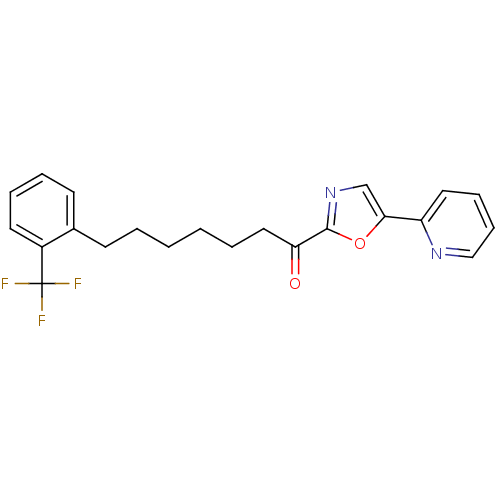 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[2-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.5 | 30 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23103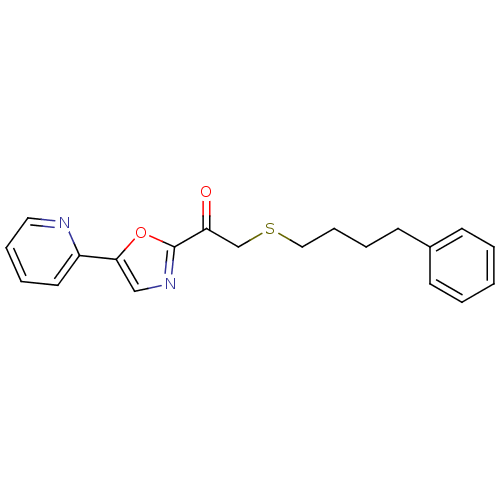 (2-[(4-phenylbutyl)sulfanyl]-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23049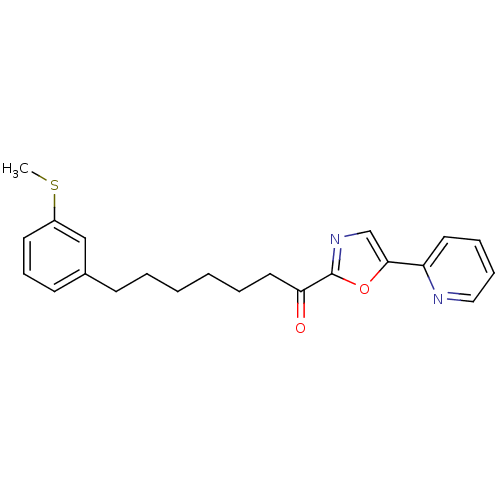 (7-[3-(methylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | -47.3 | 10 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23027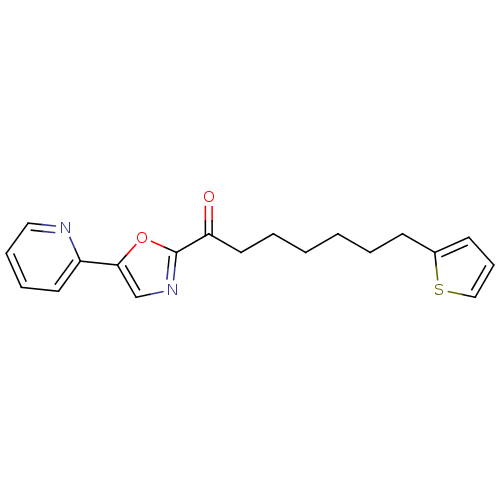 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(thiophen-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | -47.3 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23120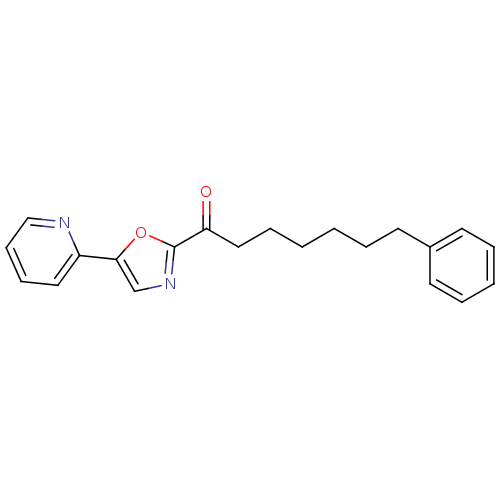 (7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | -47.5 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23090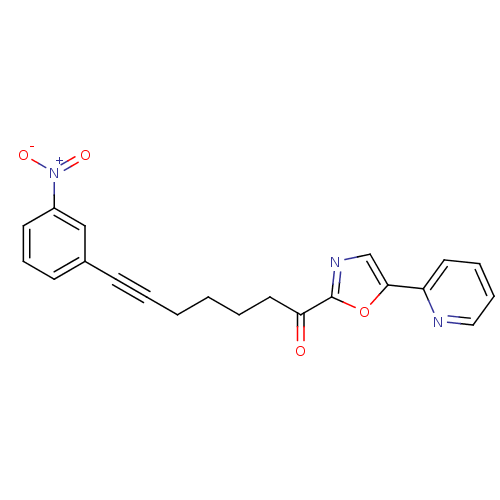 (7-(3-nitrophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | -47.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23101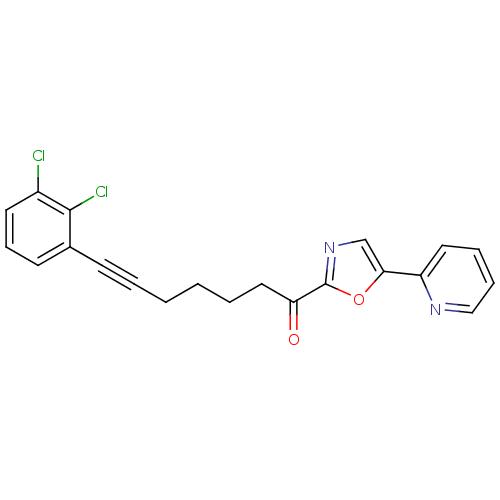 (7-(2,3-dichlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23029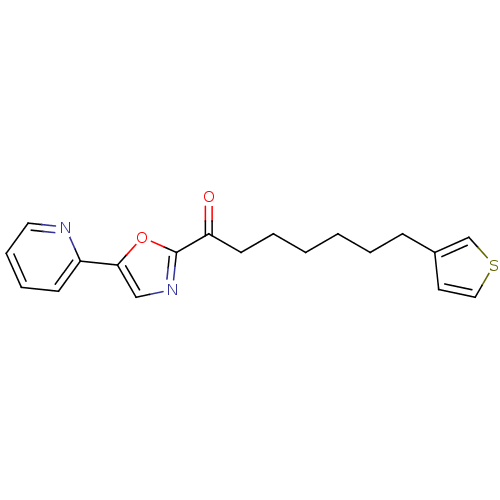 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(thiophen-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.10 | -46.9 | 40 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23044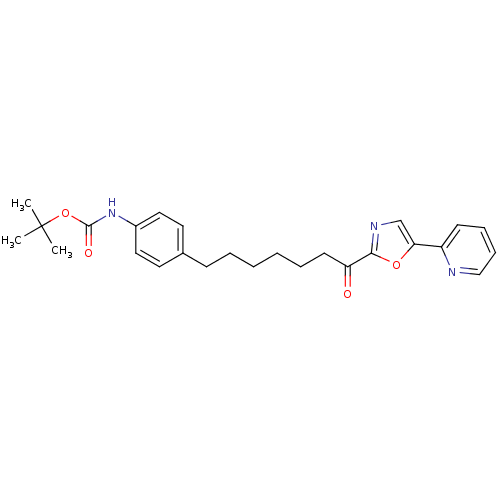 (alpha-ketooxazole, 5p | tert-butyl N-(4-{7-oxo-7-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60 | -46.6 | n/a | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23038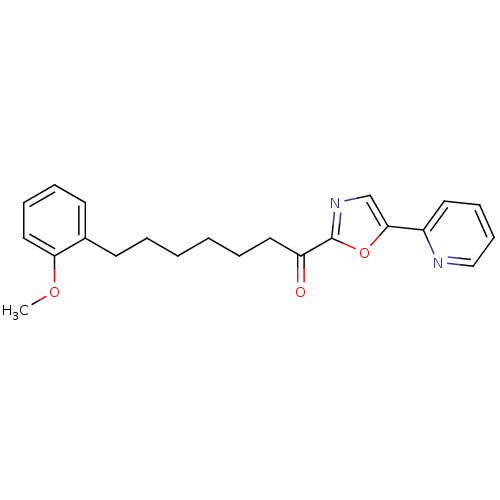 (7-(2-methoxyphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | -46.5 | 10 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23040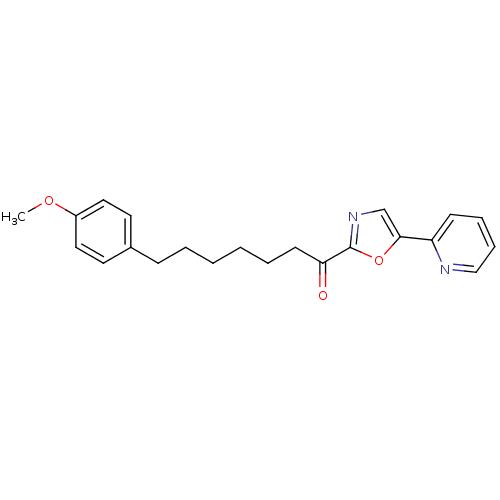 (7-(4-methoxyphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.20 | -46.4 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23051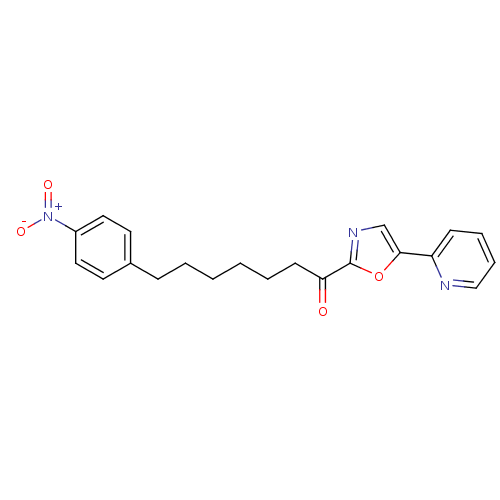 (7-(4-nitrophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.5 | -46.3 | 0.5 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23067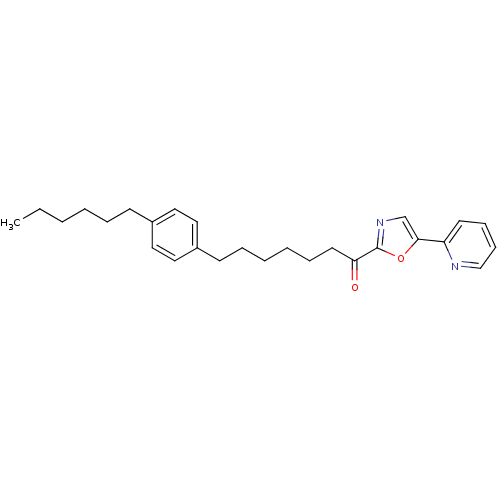 (7-(4-hexylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.70 | -46.7 | 30 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23099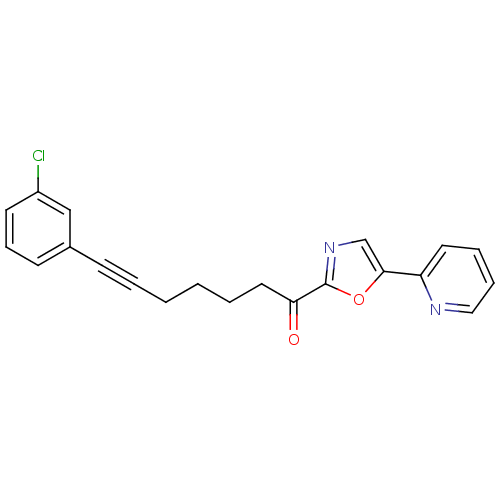 (7-(3-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23124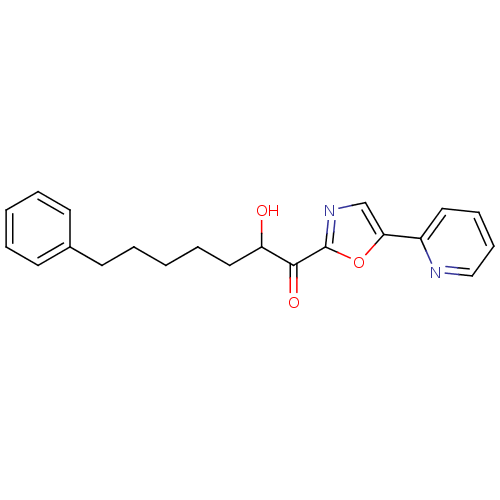 (2-hydroxy-7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23086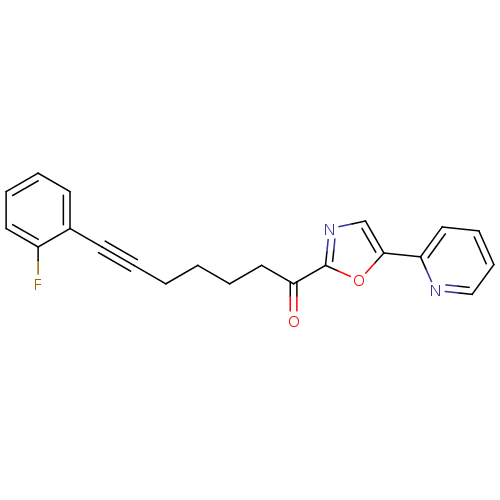 (7-(2-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23093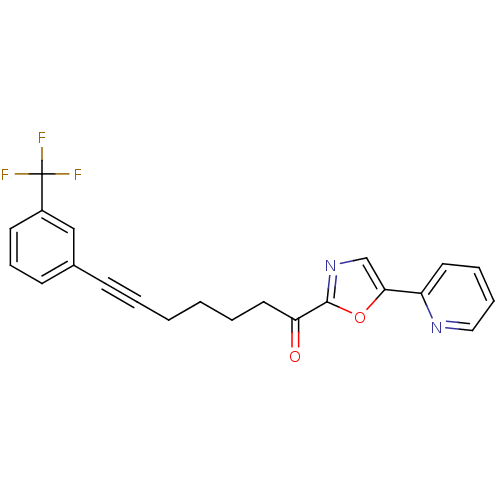 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[3-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | -45.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23031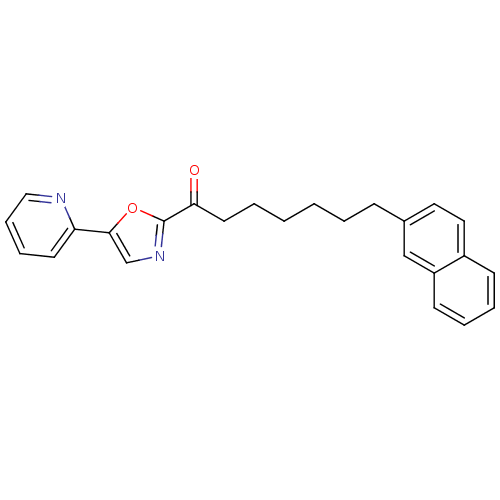 (7-(naphthalen-2-yl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | -45.0 | 40 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23084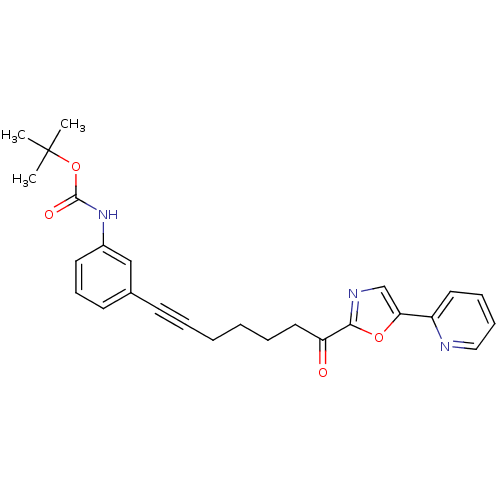 (alpha-ketooxazole, 4o | tert-butyl N-(3-{7-oxo-7-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23098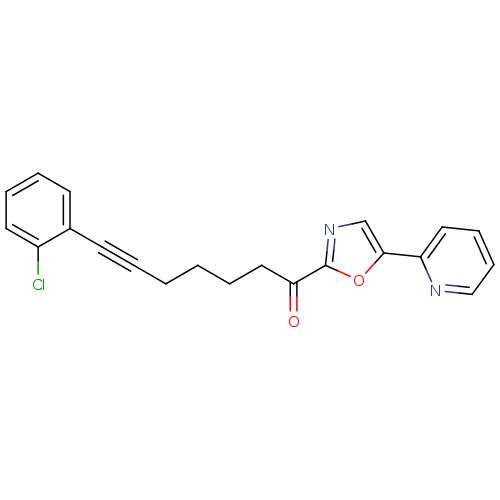 (7-(2-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23115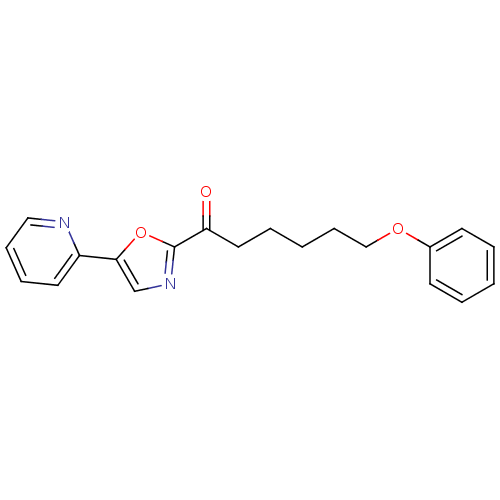 (6-phenoxy-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hexa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | -44.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23068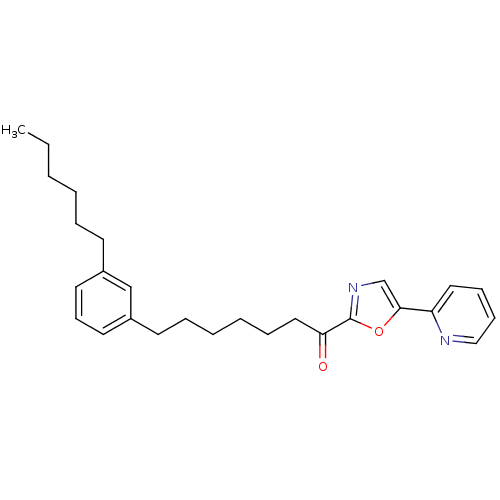 (7-(3-hexylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -44.7 | 80 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23092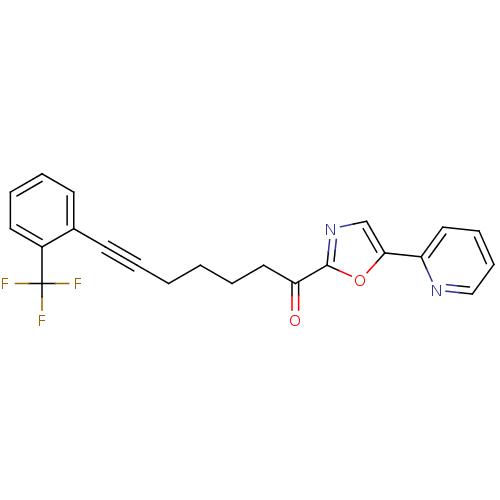 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[2-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | -44.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23089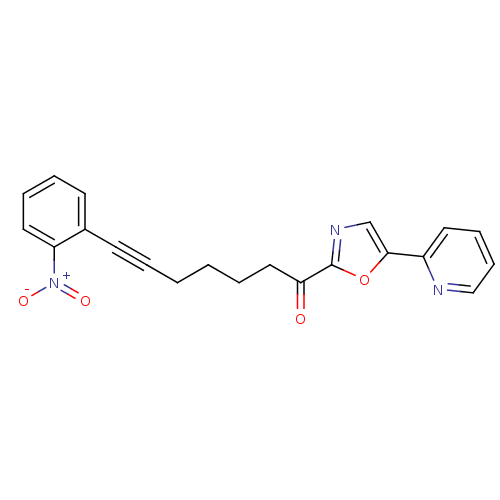 (7-(2-nitrophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | -44.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23066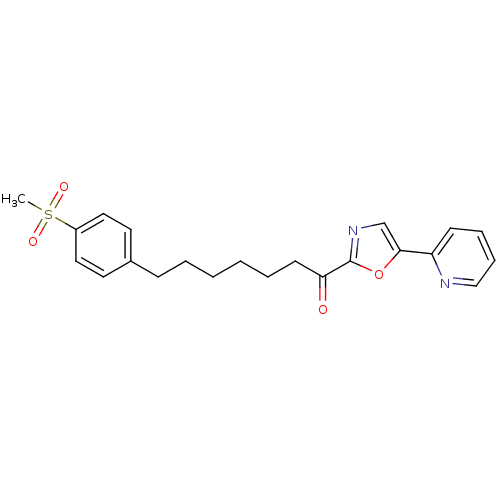 (7-(4-methanesulfonylphenyl)-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | -44.1 | 50 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23100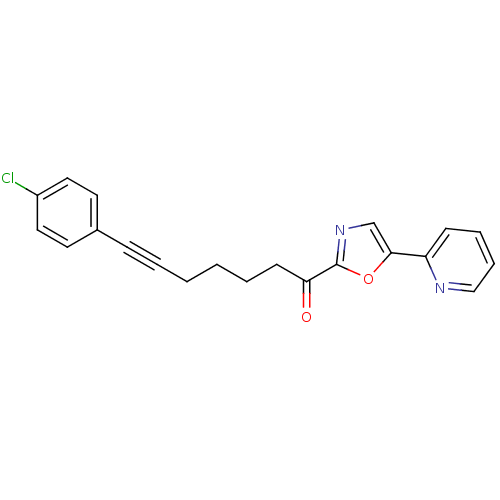 (7-(4-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23085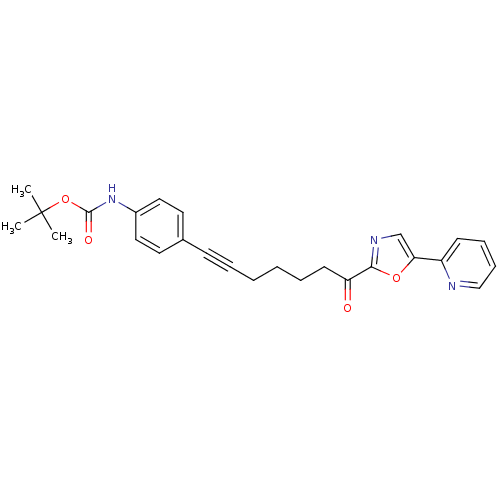 (alpha-ketooxazole, 4p | tert-butyl N-(4-{7-oxo-7-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | -43.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23071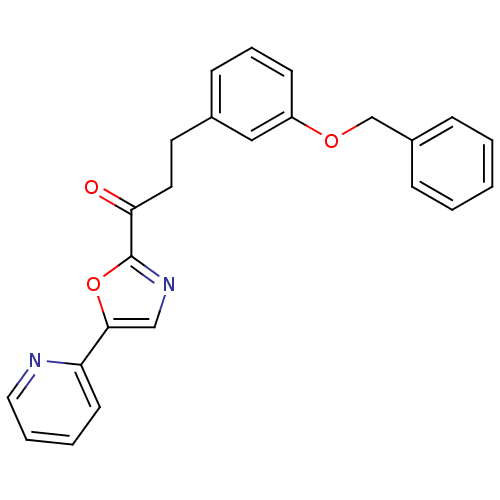 (3-[3-(benzyloxy)phenyl]-1-[5-(pyridin-2-yl)-1,3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.4 | 100 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23097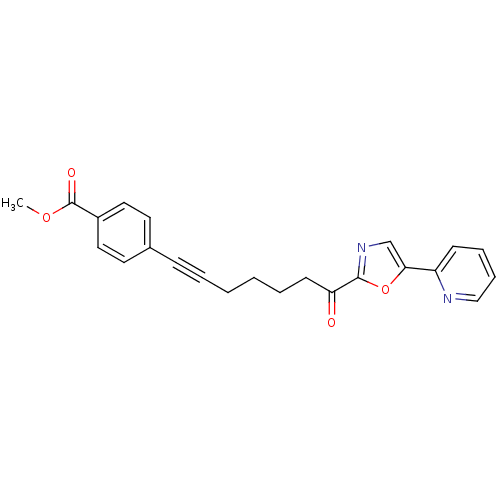 (alpha-ketooxazole, 4cc | methyl 4-{7-oxo-7-[5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23112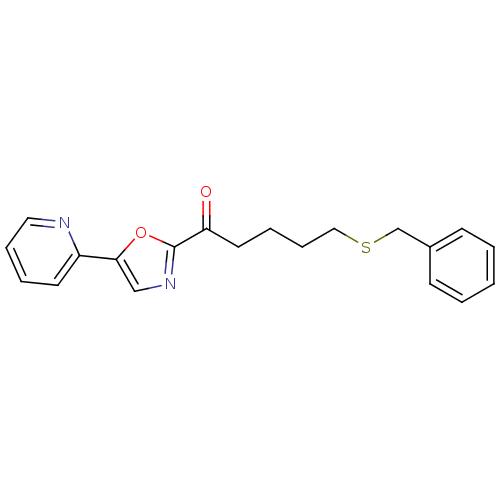 (5-(benzylsulfanyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23083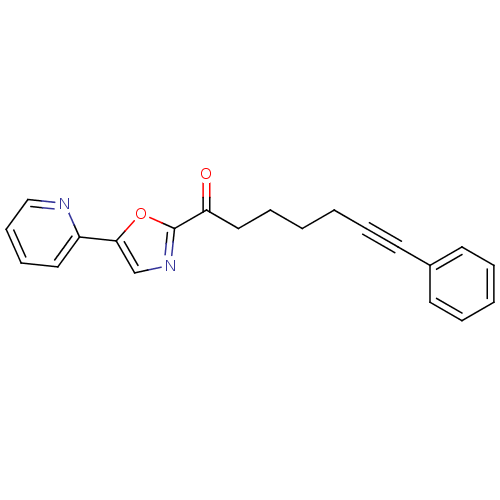 (7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hept-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | -43.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23041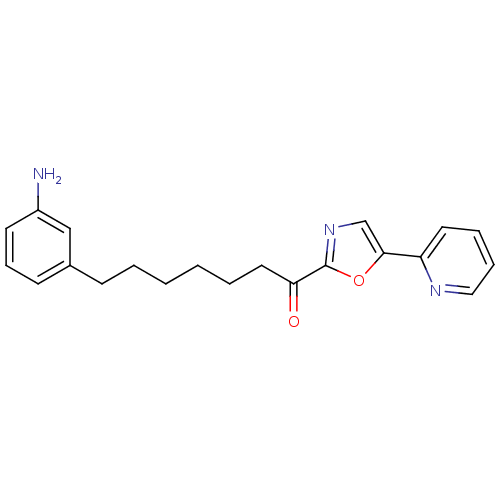 (7-(3-aminophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23096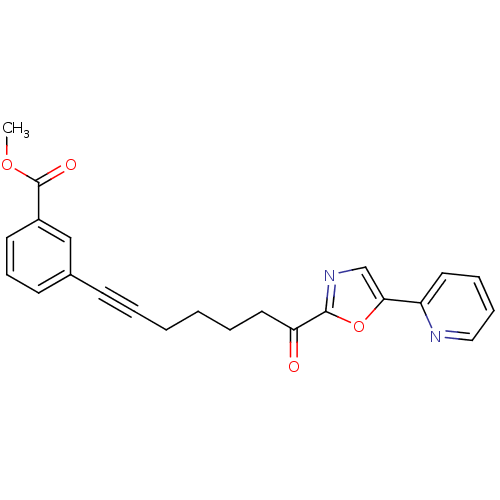 (alpha-ketooxazole, 4bb | methyl 3-{7-oxo-7-[5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23091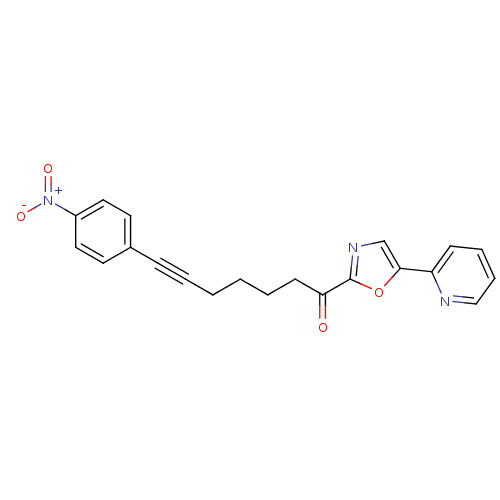 (7-(4-nitrophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | -42.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23033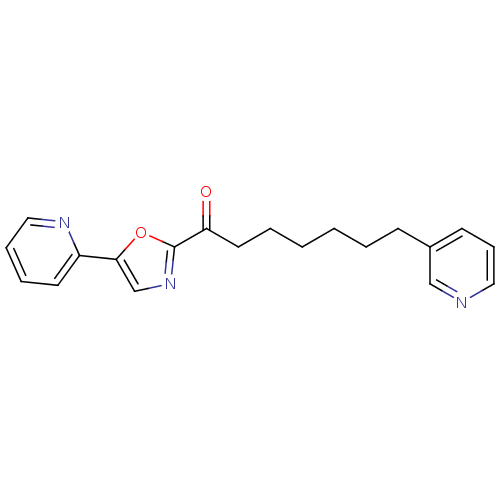 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(pyridin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | -42.3 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23095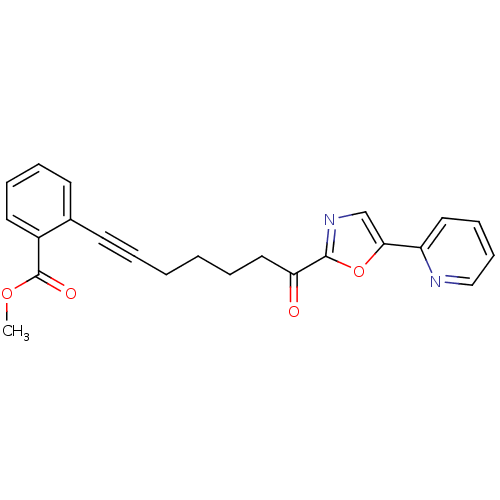 (alpha-ketooxazole, 4aa | methyl 2-{7-oxo-7-[5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 34 | -42.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23129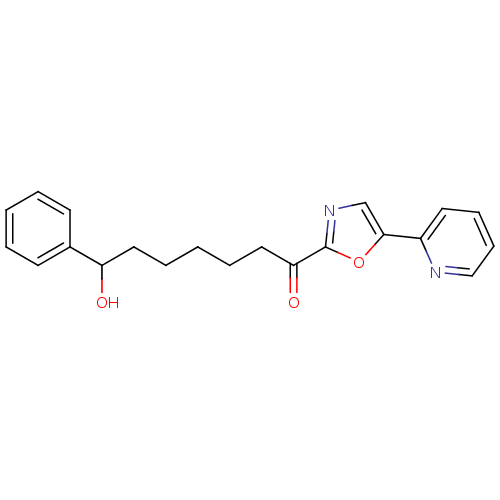 (7-hydroxy-7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | -42.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23125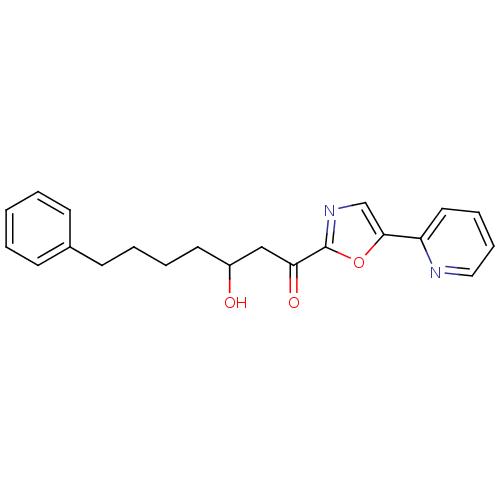 (3-hydroxy-7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | -42.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23088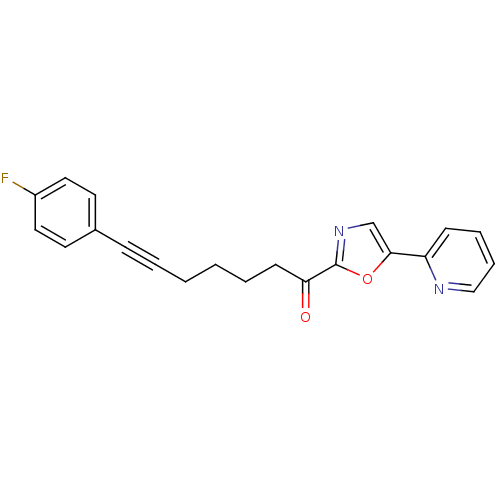 (7-(4-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | -42.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23064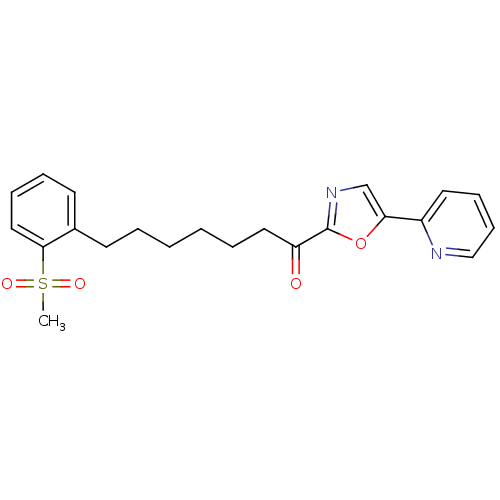 (7-(2-methanesulfonylphenyl)-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | -42.4 | 100 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23116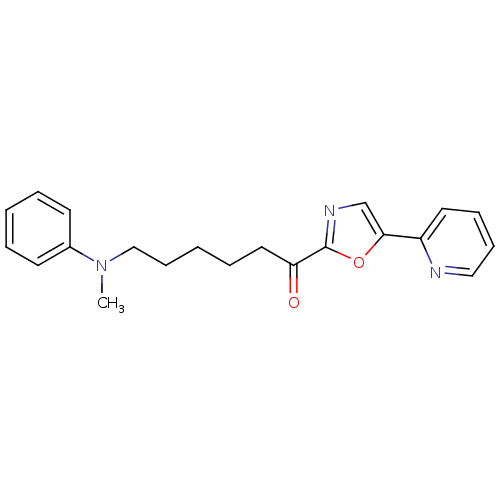 (6-[methyl(phenyl)amino]-1-[5-(pyridin-2-yl)-1,3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23110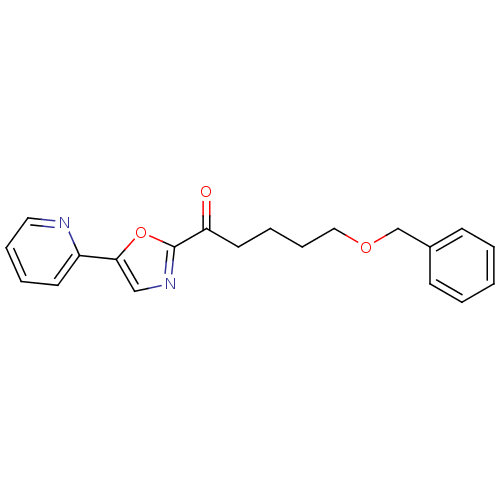 (5-(benzyloxy)-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | -41.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23102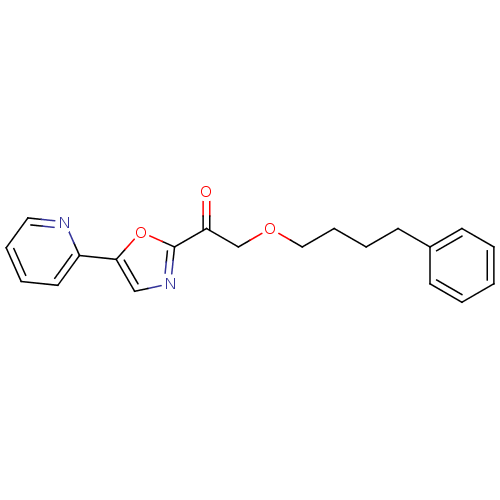 (2-(4-phenylbutoxy)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | -41.2 | 10 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23108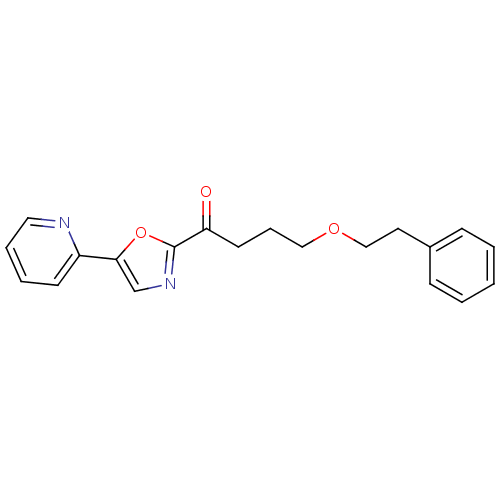 (4-(2-phenylethoxy)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 63 | -41.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23094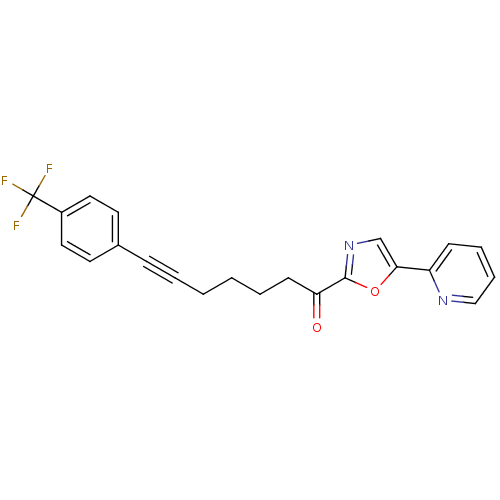 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[4-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 75 | -40.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23106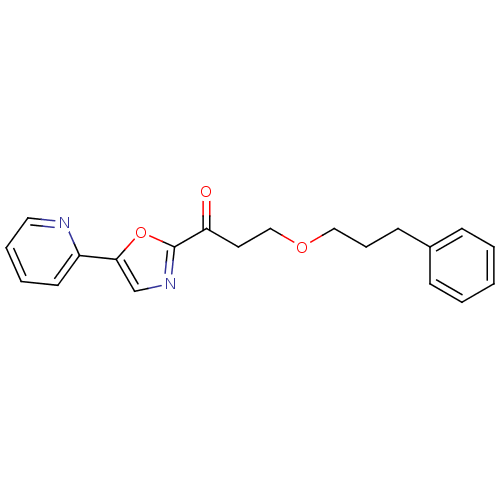 (3-(3-phenylpropoxy)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 90 | -40.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23032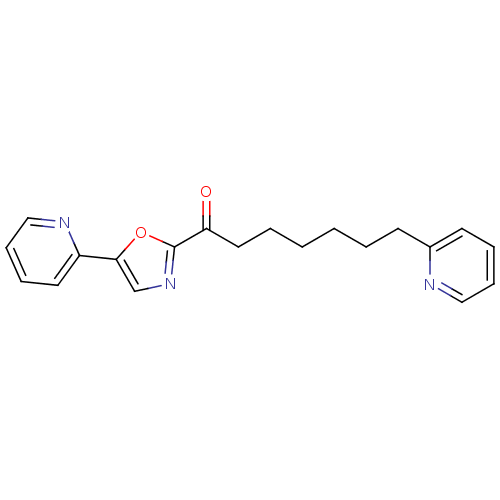 (7-(pyridin-2-yl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | -39.1 | 30 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23082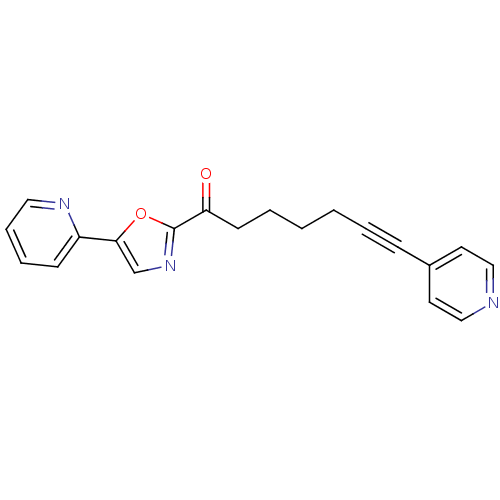 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(pyridin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | -38.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23107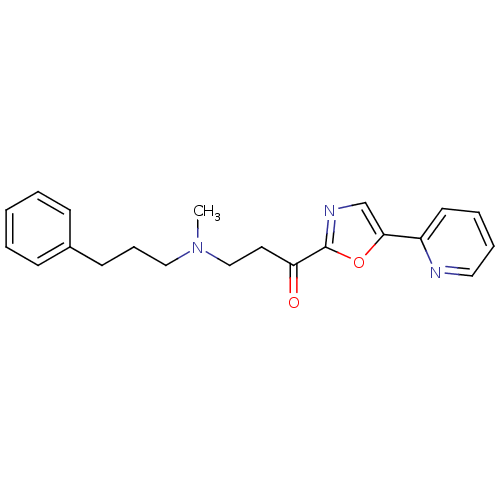 (3-[methyl(3-phenylpropyl)amino]-1-[5-(pyridin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23111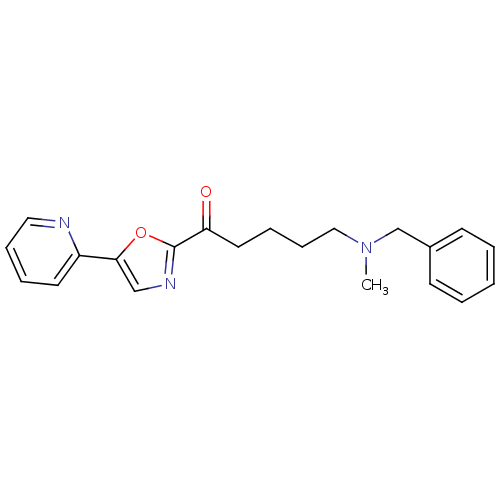 (5-[benzyl(methyl)amino]-1-[5-(pyridin-2-yl)-1,3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23128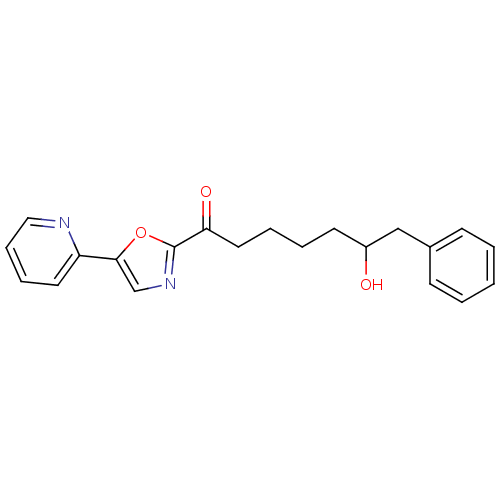 (6-hydroxy-7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | -38.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23080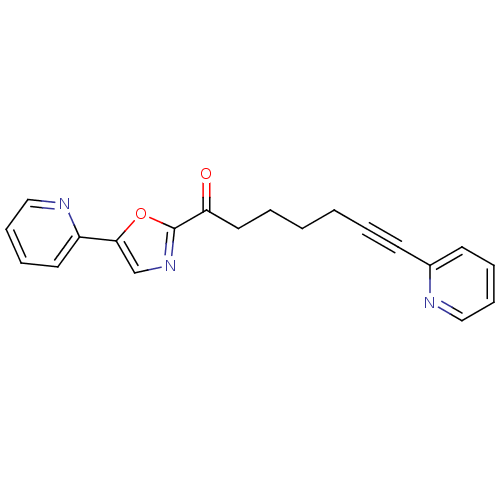 (7-(pyridin-2-yl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | -37.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23081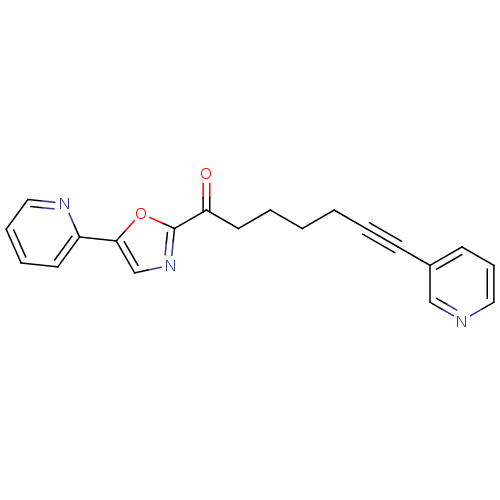 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(pyridin-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | -37.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23126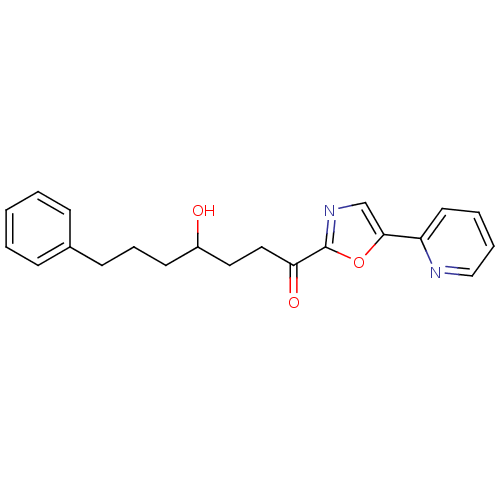 (4-hydroxy-7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | -36.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23058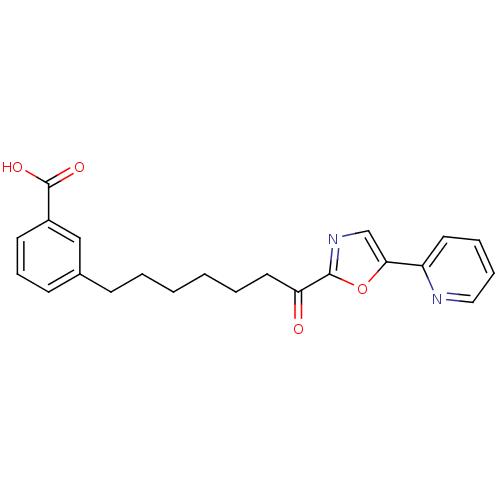 (3-{7-oxo-7-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >600 | >-35.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23035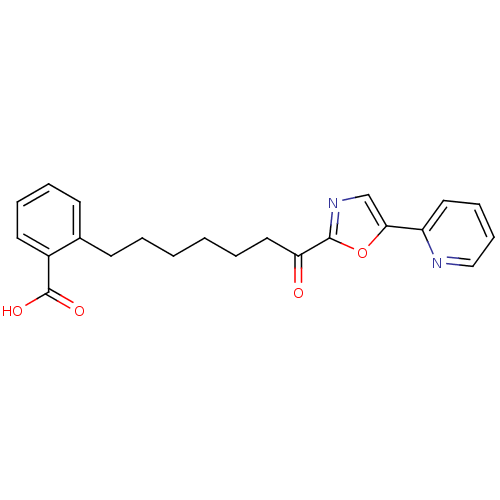 (2-{7-oxo-7-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >600 | >-35.2 | n/a | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23059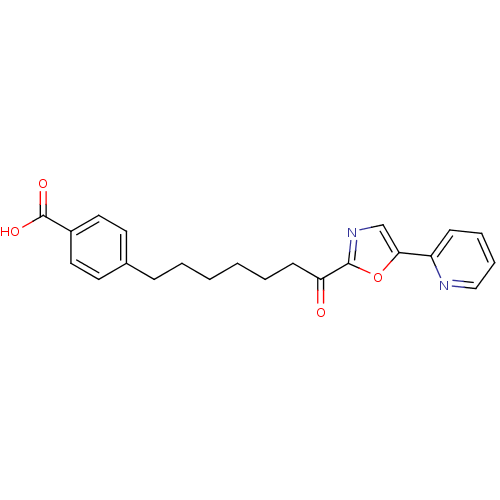 (4-{7-oxo-7-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >600 | >-35.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23135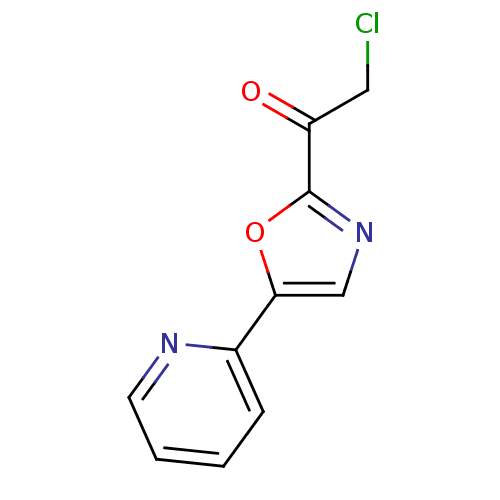 (2-chloro-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]ethan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 800 | -34.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23104 (2-[(4-phenylbutane)sulfinyl]-1-[5-(pyridin-2-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23118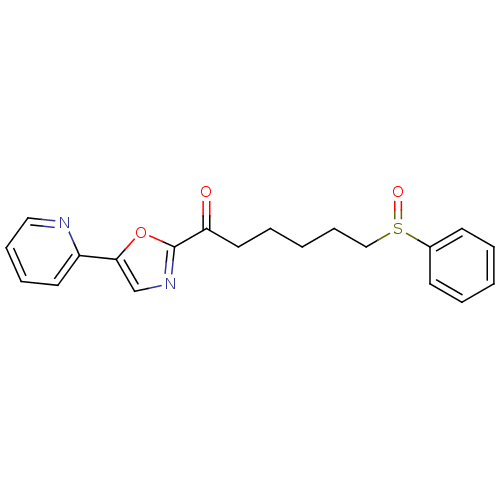 (6-(benzenesulfinyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23127 (5-hydroxy-7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23132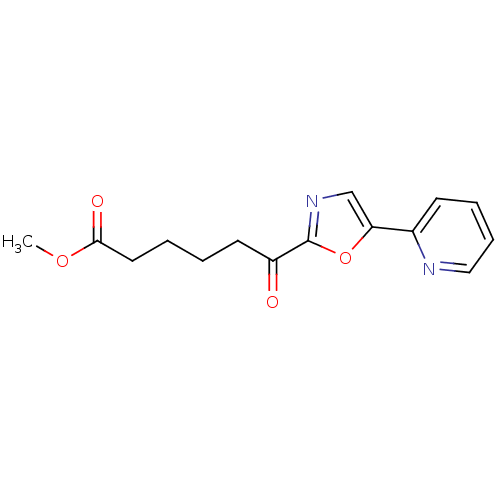 (alpha-ketooxazole, 14c | methyl 6-oxo-6-[5-(pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+3 | -32.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23136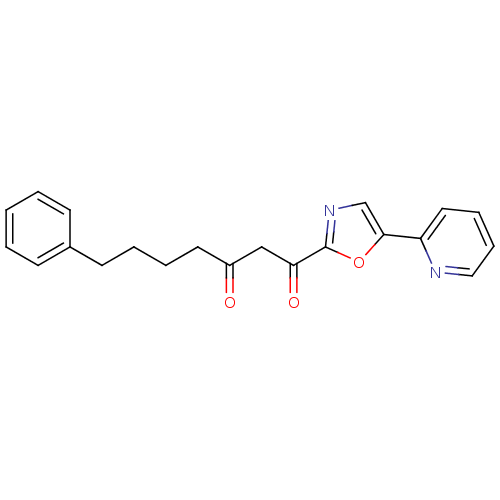 (7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+3 | >-32.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23137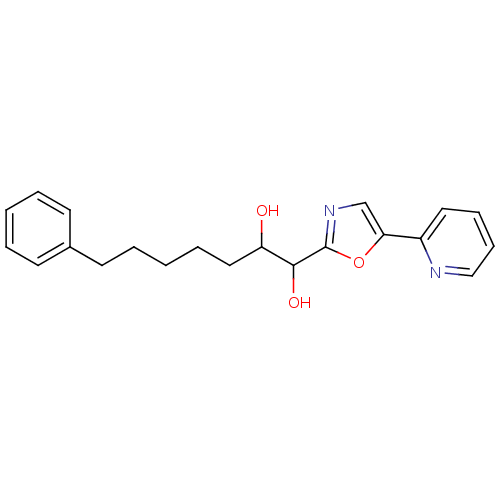 (7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23072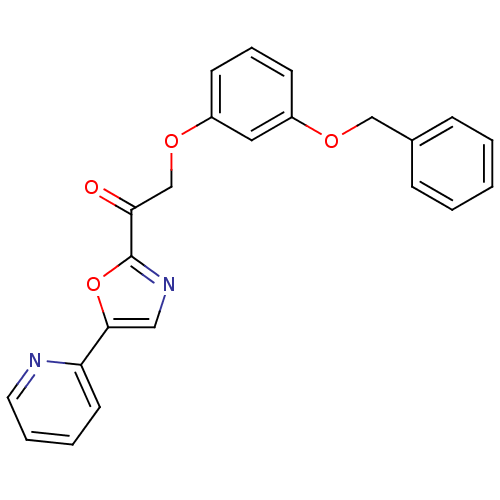 (2-[3-(benzyloxy)phenoxy]-1-[5-(pyridin-2-yl)-1,3-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | -32.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23113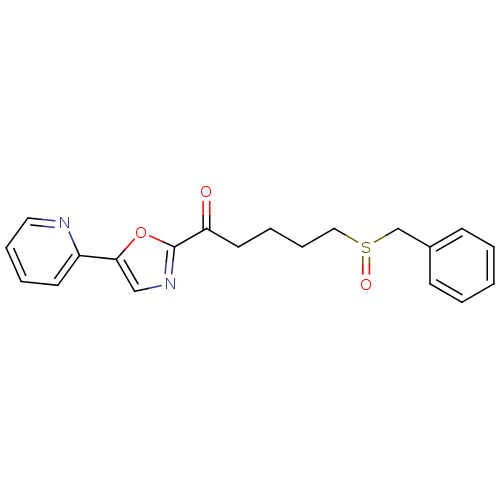 (5-(phenylmethane)sulfinyl-1-[5-(pyridin-2-yl)-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23134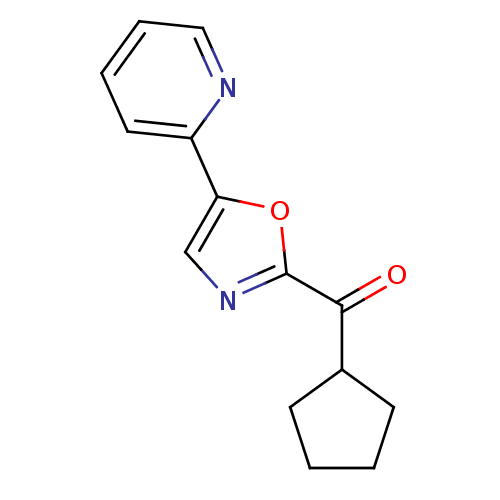 (2-cyclopentanecarbonyl-5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23109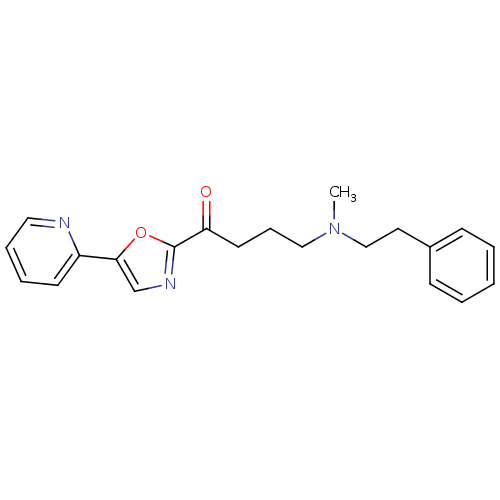 (4-[methyl(2-phenylethyl)amino]-1-[5-(pyridin-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23123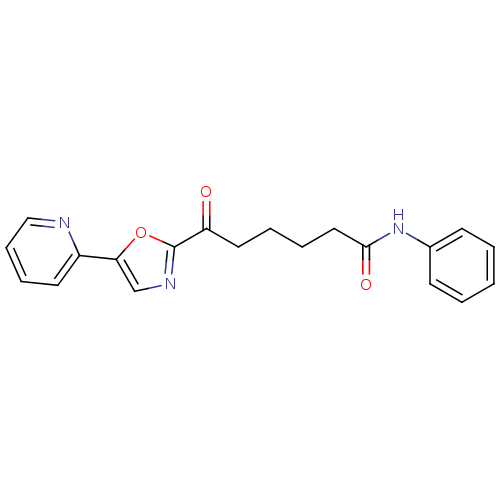 (6-oxo-N-phenyl-6-[5-(pyridin-2-yl)-1,3-oxazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10E+3 | -31.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23070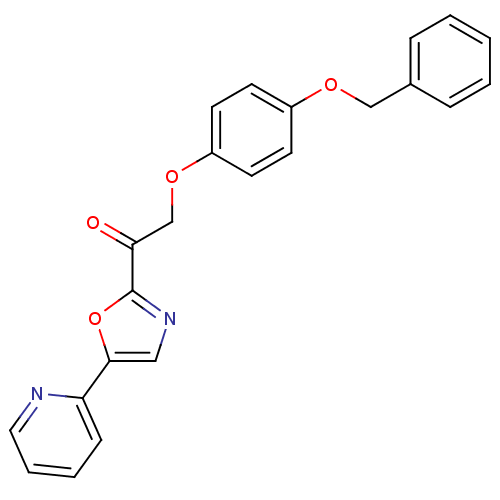 (2-[4-(benzyloxy)phenoxy]-1-[5-(pyridin-2-yl)-1,3-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30E+3 | -30.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23119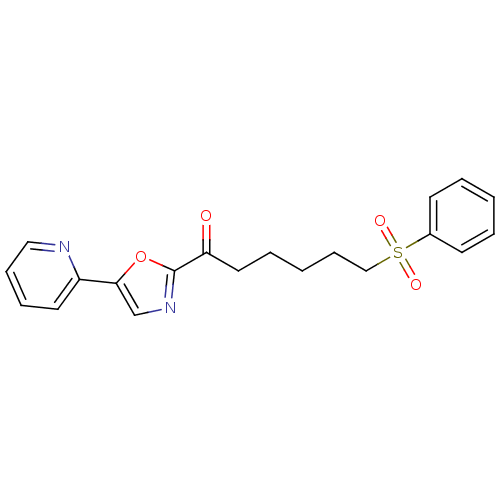 (6-(benzenesulfonyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23131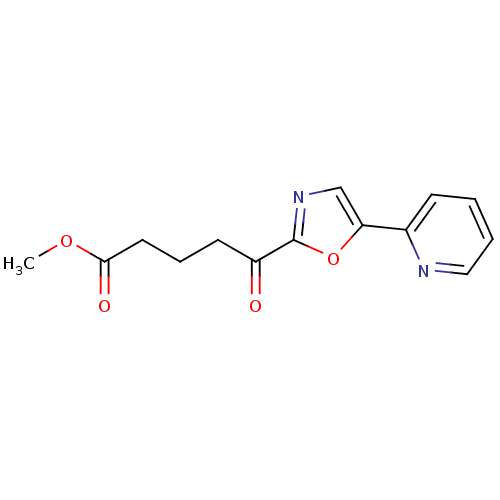 (alpha-ketooxazole, 14b | methyl 5-oxo-5-[5-(pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 7.00E+3 | -29.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23114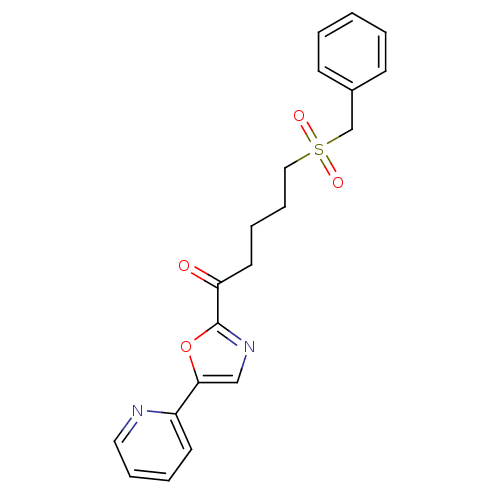 (5-(phenylmethane)sulfonyl-1-[5-(pyridin-2-yl)-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23105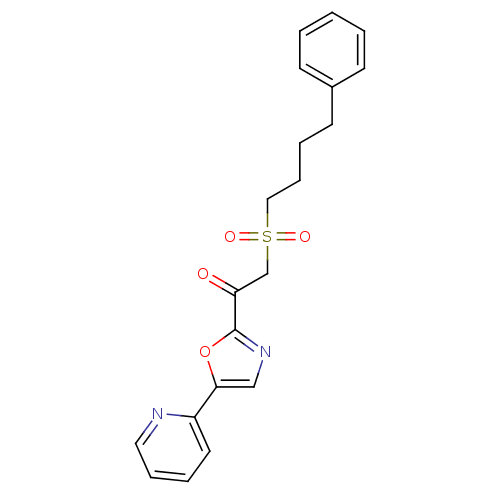 (2-[(4-phenylbutane)sulfonyl]-1-[5-(pyridin-2-yl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+4 | -26.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23122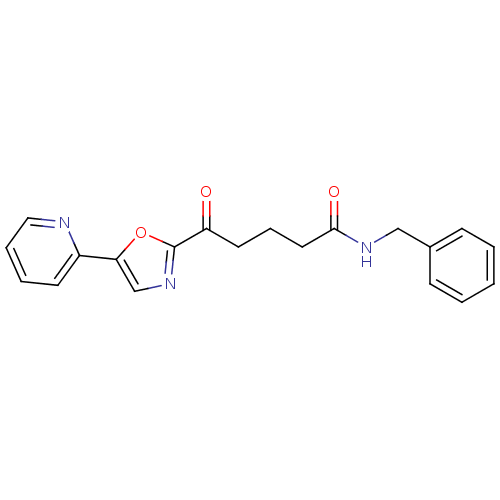 (N-benzyl-5-oxo-5-[5-(pyridin-2-yl)-1,3-oxazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+4 | -26.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23133 (2-cyclopropanecarbonyl-5-(pyridin-2-yl)-1,3-oxazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | >-24.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23121 (4-oxo-N-(2-phenylethyl)-4-[5-(pyridin-2-yl)-1,3-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | >-22.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23130 (alpha-ketooxazole, 14a | methyl 4-oxo-4-[5-(pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+5 | >-22.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23138 (2-[methyl(4-phenylbutyl)amino]-1-[5-(pyridin-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | >1.00E+5 | >-22.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23031 (7-(naphthalen-2-yl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23079 (3-[4-(benzyloxy)phenyl]-1-{pyrido[2,3-d][1,3]oxazo...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23077 (3-[4-(phenylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23053 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[3-(trifluo...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23074 (3-(4-phenoxyphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23054 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[4-(trifluo...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23030 (7-(naphthalen-1-yl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23049 (7-[3-(methylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23102 (2-(4-phenylbutoxy)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23045 (7-(2-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23061 (7-(3-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23063 (7-(2,3-dichlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxa...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23120 (7-phenyl-1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]hepta...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23060 (7-(2-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23075 (3-[4-(phenylamino)phenyl]-1-[5-(pyridin-2-yl)-1,3-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23027 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(thiophen-2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23029 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(thiophen-3...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23050 (7-[4-(methylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23076 (3-(4-benzylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23032 (7-(pyridin-2-yl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23046 (7-(3-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23033 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-(pyridin-3-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23039 (7-(3-methoxyphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23047 (7-(4-fluorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23078 (3-(4-phenylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23062 (7-(4-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23071 (3-[3-(benzyloxy)phenyl]-1-[5-(pyridin-2-yl)-1,3-ox...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23073 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23066 (7-(4-methanesulfonylphenyl)-1-[5-(pyridin-2-yl)-1,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23064 (7-(2-methanesulfonylphenyl)-1-[5-(pyridin-2-yl)-1,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23042 (7-(4-aminophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23034 (7-(2-methylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23065 (7-(3-methanesulfonylphenyl)-1-[5-(pyridin-2-yl)-1,...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23037 (7-(4-methylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23040 (7-(4-methoxyphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23036 (7-(3-methylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23038 (7-(2-methoxyphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23057 (alpha-ketooxazole, 5cc | methyl 4-{7-oxo-7-[5-(pyr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23052 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[2-(trifluo...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23069 (3-[4-(benzyloxy)phenyl]-1-[5-(pyridin-2-yl)-1,3-ox...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23048 (7-[2-(methylsulfanyl)phenyl]-1-[5-(pyridin-2-yl)-1...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23056 (alpha-ketooxazole, 5bb | methyl 3-{7-oxo-7-[5-(pyr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23055 (alpha-ketooxazole, 5aa | methyl 2-{7-oxo-7-[5-(pyr...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23068 (7-(3-hexylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23067 (7-(4-hexylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Homo sapiens (Human)) | BDBM23051 (7-(4-nitrophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-2...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description Inhibition of TGH activity was assayed using COS-7 expressed TGH and the chromogenic substrate. IC50 values were determined from the inhibition obser... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||